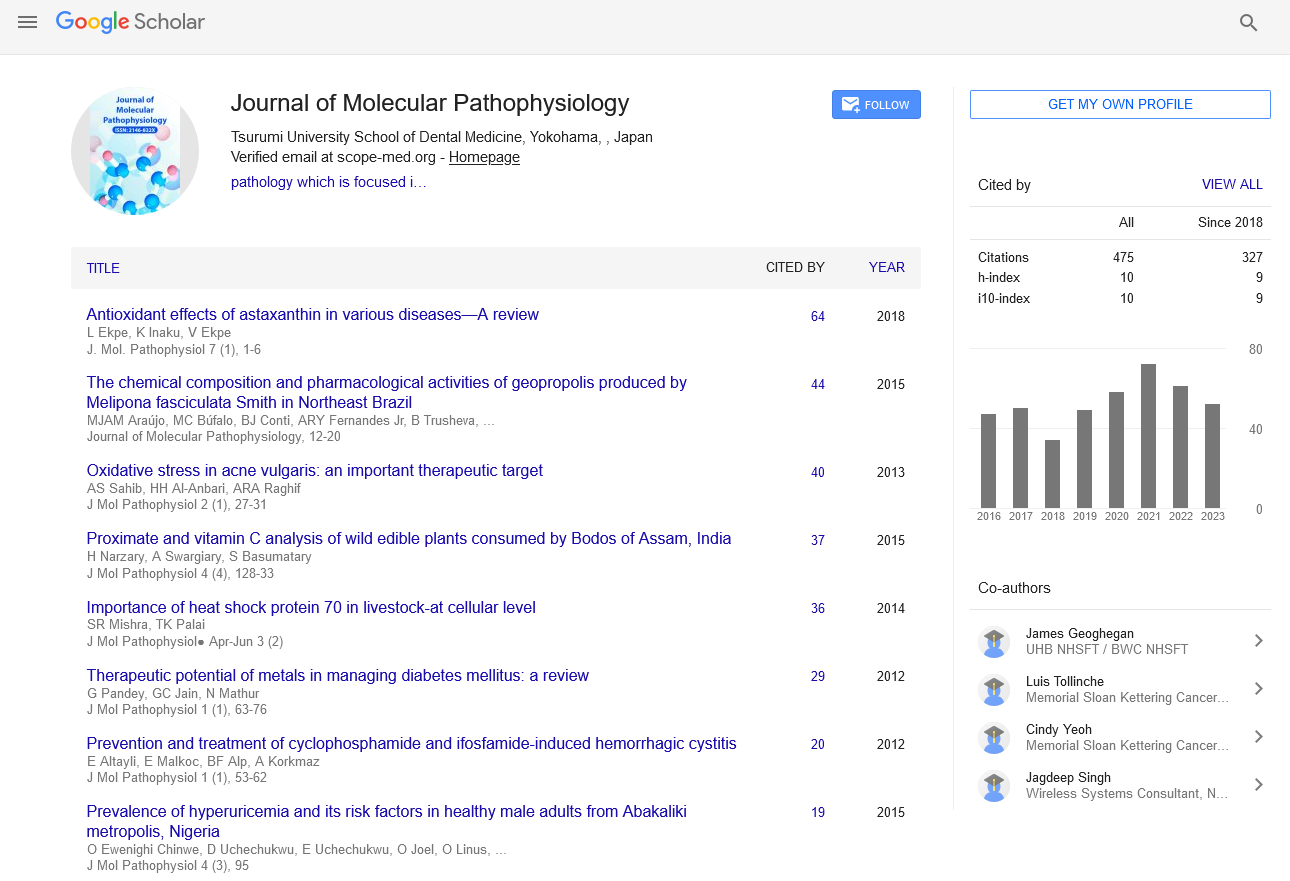
Opinion - Journal of Molecular Pathophysiology (2023)
The Mechanisms in Acute Ischemic Stroke
Sophie Lijdsman*Sophie Lijdsman, Department of Pathology,, Ningxia Medical University, Yinchuan, China, Email: Lijdsmans12345@yahoo.com
Received: 19-Jun-2023, Manuscript No. JMOLPAT-23-106882; Editor assigned: 22-Jun-2023, Pre QC No. JMOLPAT-23-106882 (PQ); Reviewed: 07-Jul-2023, QC No. JMOLPAT-23-106882; Revised: 14-Jul-2023, Manuscript No. JMOLPAT-23-106882 (R); Published: 21-Jul-2023
About the Study
Acute ischemic stroke is a devastating neurological condition characterized by the sudden interruption of blood supply to a specific area of the brain, leading to the impairment of neuronal function. This interruption occurs due to the occlusion or blockage of a cerebral blood vessel, resulting in the lack of oxygen and nutrients necessary for the normal functioning of brain tissue. The pathophysiology of acute ischemic stroke involves a complex cascade of events that ultimately lead to neuronal injury and subsequent functional deficits.
The primary event in acute ischemic stroke is the development of cerebral ischemia, which occurs when blood flow to the brain is compromised. Ischemia can arise from different mechanisms, including thrombotic or embolic occlusion of a cerebral artery or systemic hypo perfusion. The reduction in blood flow leads to a decline in the supply of oxygen and glucose to brain tissue, initiating a series of cellular and molecular events. The lack of oxygen and glucose supply results in impaired energy metabolism within brain cells. The brain’s high demand for energy makes it particularly susceptible to energy failure. In the absence of energy production through oxidative phosphorylation, anaerobic glycolysis becomes the primary source of ATP production. However, anaerobic glycolysis is less efficient and leads to the accumulation of lactate, causing a drop in pH and further cellular dysfunction.
Ischemia triggers a massive release of excitatory neurotransmitters, primarily glutamate, into the extracellular space. The excess glutamate over activates postsynaptic glutamate receptors, particularly N-Methyl-D-Aspartate (NMDA) receptors, leading to a surge of intracellular calcium influx. The intracellular calcium overload triggers several damaging pathways, including activation of proteases, lipases, and nitric oxide synthase, resulting in the generation of reactive oxygen species and oxidative stress.
The excessive release of glutamate and subsequent calcium influx activates various enzymes, including NADPH oxidase and nitric oxide synthase, leading to the production of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS). ROS and RNS cause oxidative damage to cellular components, including lipids, proteins, and DNA. The accumulation of free radicals further exacerbates cellular injury and inflammation, contributing to the progression of ischemic brain damage. Ischemic stroke triggers an inflammatory response within the affected brain tissue. The release of pro-inflammatory cytokines, such as Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-1 beta (IL-1β), promotes the activation and recruitment of immune cells, including microglia and peripheral leukocytes. Microglia, the resident immune cells in the brain, become activated and release additional pro-inflammatory mediators, exacerbating the inflammatory cascade. The inflammatory response further amplifies neuronal injury and contributes to the breakdown of the blood-brain barrier. Ischemic stroke leads to the disruption of the Blood- Brain Barrier (BBB), which normally protects the brain from circulating toxins and immune cells. The inflammatory response, combined with oxidative stress, triggers the breakdown of tight junction proteins, allowing the infiltration of immune cells and circulating molecules into the brain parenchyma. The influx of immune cells and neurotoxic substances intensifies the inflammatory response and amplifies neuronal damage. Prolonged ischemia and the subsequent cascade of events lead to cellular apoptosis and necrosis. Apoptosis, or programmed cell death, is triggered by multiple pathways, including the activation of caspases, release of cytochrome c, and DNA fragmentation. Necrotic cell death occurs due to the rapid loss of cellular energy and the inability to maintain cell membrane integrity. Both apoptotic and necrotic cell death contribute to the expansion of the infarcted area and the loss of functional brain tissue.
Acute ischemic stroke is a complex disorder involving a multitude of pathophysiologic mechanisms. Cerebral ischemia initiates a cascade of events, including energy failure, excitotoxicity, oxidative stress, inflammation, blood-brain barrier disruption, and apoptotic and necrotic cell death. Understanding these mechanisms is crucial for the development of targeted therapeutic interventions aimed at preserving brain tissue and improving functional outcomes in patients with acute ischemic stroke. Further research and advancements in this field hold the potential to enhance stroke management and reduce the burden of this debilitating condition on individuals and society as a whole.
Copyright: © 2023 The Authors. This is an open access article under the terms of the Creative Commons Attribution Non Commercial Share Alike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.