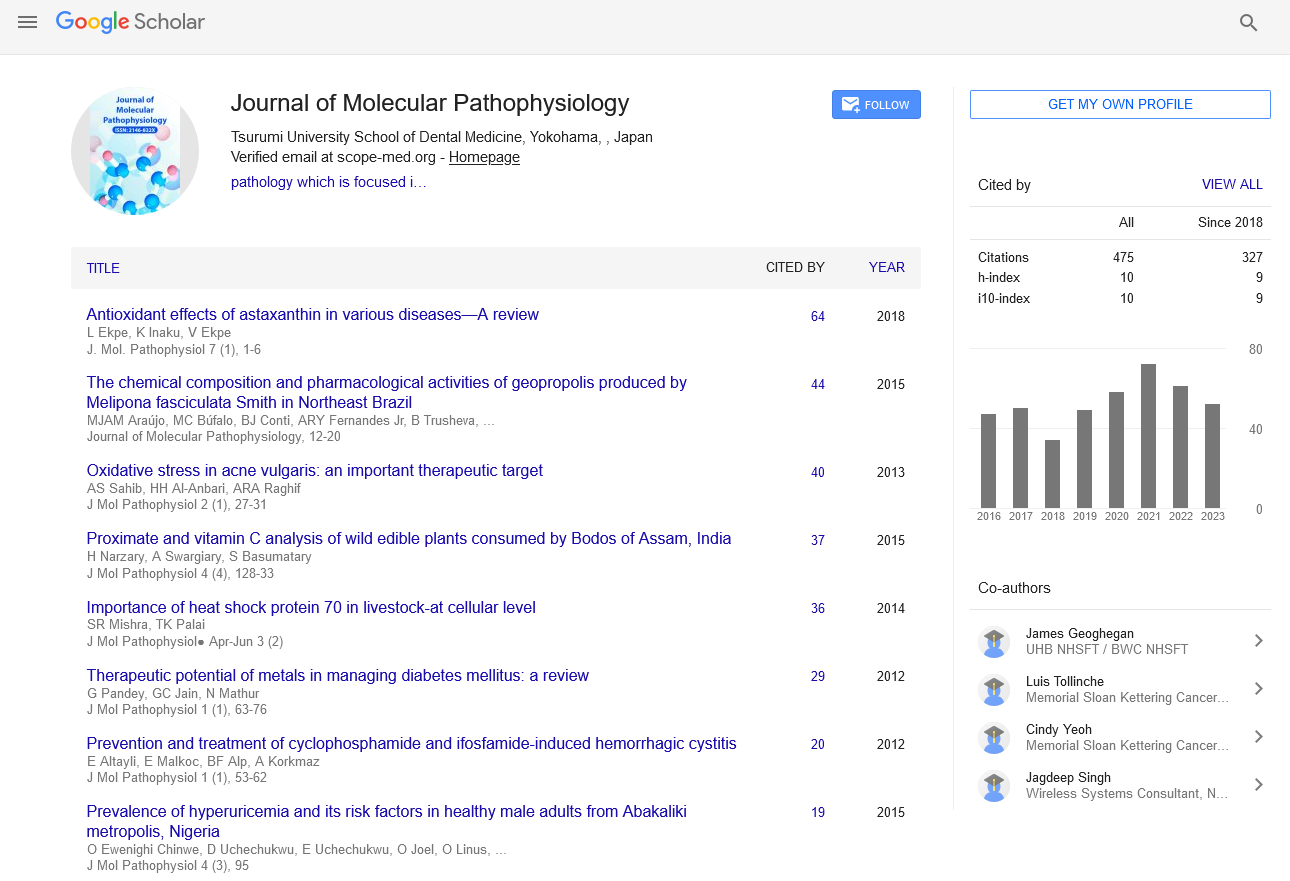
Perspective - Journal of Molecular Pathophysiology (2024)
Mechanisms of Autoantibody-Mediated Cell Destruction in Systemic Lupus Erythematosus
Carlos Harrell*Carlos Harrell, Department of Immunology, National University of Colombia, Bogota, Colombia, Email: harrellcarlo2020@yahoo.com
Received: 10-Jun-2024, Manuscript No. JMOLPAT-24-144395; Editor assigned: 13-Jun-2024, Pre QC No. JMOLPAT-24-144395 (PQ); Reviewed: 28-Jun-2024, QC No. JMOLPAT-24-144395; Revised: 05-Jul-2024, Manuscript No. JMOLPAT-24-144395 (R); Published: 12-Jul-2024
About the Study
Systemic Lupus Erythematosus (SLE) is an autoimmune disease characterized by a loss of tolerance to self-antigens, resulting in widespread inflammation and tissue damage. The immune system, which typically distinguishes between self and non-self, fails to maintain this discrimination, leading to autoimmunity. Central to this process is the aberrant activation of auto reactive B cells and T cells. Auto reactive B cells produce autoantibodies against nuclear components such as DNA, histones, and ribonucleoproteins. These autoantibodies form immune complexes with their antigens, which develop in different tissues, initiating inflammation and tissue damage through the activation of complement pathways and recruitment of inflammatory cells.
The vulnerability to SLE is mostly influenced by genetic factors. The disease has been related to a large number of genes, many of which are involved in the control of immunological responses. Variations in genes encoding components of the complement system, fragment crystallizable (Fc) receptors, and molecules involved in T and B cell signaling pathways are associated with increased risk of SLE. Moreover, polymorphisms in the Human Leukocyte Antigen- DR (HLA-DR) and Human Leukocyte Antigen-DQ (HLA-DQ) regions have been strongly related to SLE, emphasizing the importance of antigen presentation in the disease’s pathogenesis.
Environmental factors also contribute to the beginning and exacerbation of SLE. Ultra Violet (UV) light induces cell damage and apoptosis, leading to the release of nuclear antigens. In genetically predisposed individuals, the clearance of apoptotic cells is impaired, resulting in the accumulation of cellular debris and increased availability of auto antigens. This process fosters the formation of immune complexes and continues the autoimmune response. Infections, particularly viral infections, have been implicated in the initiation and exacerbation of SLE. The production of type I interferons (IFNs) is a feature of SLE. plasmacytoid Dendritic Cells (pDCs) are a major source of type I IFNs in SLE. These cells recognize nucleic acids from apoptotic cells through Toll-Like Receptors (TLRs), leading to the production of IFNs. Type I IFNs play an important role in increasing the autoimmune response by enhancing the activation and survival of auto reactive B cells and allowing T helper cell development into proinflammatory subtypes. Elevated levels of type I IFNs are associated with increased disease activity and severity in SLE.
B cells and the autoantibodies they produce are important to the pathophysiology of SLE. In addition to their role in forming immune complexes, autoantibodies can directly bind to cell surface antigens, leading to cell destruction through mechanisms such as Antibody-Dependent Cellular Cytotoxicity (ADCC) and complement-mediated lysis. The formation of Neutrophil Extracellular Traps (NETs) is another mechanism through which autoantibodies contribute to tissue damage. NETs are web-like structures composed of DNA and antimicrobial proteins released by neutrophils in response to infections and inflammatory stimuli. In SLE, autoantibodies can stimulate the formation of NETs, which consequently might cause more tissue damage and inflammation.
The involvement of multiple organs and systems is a defining feature of SLE. The kidneys are frequently affected, resulting in lupus nephritis, a major cause of morbidity and mortality in SLE patients. Immune complex deposition in the glomeruli leads to inflammation and damage, shown as proteinuria, hematuria, and impaired renal function. The skin is another common place of involvement, with patients experiencing rashes, photosensitivity, and discoid lesions. The joints are frequently affected, with many patients experiencing non-erosive arthritis characterized by pain and swelling.
Patients are at increased risk of developing atherosclerosis, leading to cardiovascular disease, which is a leading cause of death in SLE. Chronic inflammation, immune complex deposition, and endothelial dysfunction contribute to the accelerated development of atherosclerosis. Other cardiac symptoms of SLE include myocarditis and pericarditis. Hematological abnormalities are common in SLE and can include anemia, leukopenia, and thrombocytopenia. Autoantibodies against blood cells and bone marrow suppression due to inflammation are primary mechanisms underlying these abnormalities. The nervous system can also be affected, leading to neuropsychiatric lupus. Peripheral neuropathy, psychosis, seizures, and cognitive impairment are among symptoms of this.