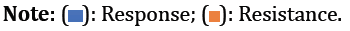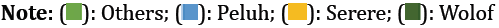Research Article - Journal of Molecular Pathophysiology (2023)
Chemotherapy Resistance and Breast Cancer in Senegal: MDR1 Gene Polymorphism Running Head: MDR1 Gene Polymorphism in Breast Cancer
Serigne Mor Samb Gueye, Fatimata Mbaye*, Mouhamadou Mansour SY and Mbacke SembeneFatimata Mbaye, Department of Animal Biology, Cheikh Anta Diop University of Dakar, Dakar, Senegal, Email: fatimata2.mbaye@ucad.edu.sn
Received: 08-Nov-2023, Manuscript No. JMOLPAT-23-119549; Editor assigned: 10-Nov-2023, Pre QC No. JMOLPAT-23-119549 (PQ); Reviewed: 27-Nov-2024, QC No. JMOLPAT-23-119549; Revised: 04-Dec-2023, Manuscript No. JMOLPAT-23-119549 (R); Published: 11-Dec-2023
Abstract
Objective: Interindividual variability in the pharmacokinetics of anticancer drugs limits their clinical use. The polymorphisms of MDR1 gene may explain these Interindividual variations and are associated with a phenotypic variation in P-glycoprotein, a membrane efflux pump that eliminates anthracyclines. This study aimed to investigate the role of two polymorphisms, C1236T and C3435T, in exons 12 and 26 of MDR1 gene in Senegalese patients with breast cancer who were treated with neoadjuvant chemotherapy with anthracyclines with fluorouracil-adriamycin-cyclophosphamide or doxorubicin-cyclophosphamide AC protocols.
Methods: PCR-RFLP was used to identify different genotypes of the C1236T and C3435T polymorphisms. Here, we evaluated five clinico-pathological parameters (age, number of therapy cures, histology, Scarff-Bloom-Richardson (SBR) histoprognostic grade and tumor stage) and ethnicity in four groups consisting of Wolofs, Peuhls, Sereres and others comprising small ethnic groups (Diola, Sarakhoule, Bambara, Maure and Soce). From March to August 2018, we examined 118 patients, of whom 58 were included. Of these 27 and 31 received chemotherapy with fluorouracil-adriamycin-cyclophosphamide and doxorubicin-cyclophosphamide, respectively.
Results: The mean age of the population was 42 ± 9 years, mainly composed of Wolofs (32.7%) and Peuhls (24.1%). Most tumors were histologically classified as IDC-NOS (48.27%), Histopathological SBR II (64%) and tumor grade IV (47.2%). The distribution of genotypic and allelic frequencies was C/C (28.57%), C/T (14.28%), T/T (57.15%), C (37.71%) and T (64.29%) for exon 12 and C/C (42.59%), C/T (37.07%), T/T (20.34%), C (67.18%), and T (32.82%) for exon 26. All these distributions follow the Hardy–Weinberg equilibrium. We obtained a 43.10% response rate. C3435T polymorphism in exon 26 of the MDR1 gene was found to be markedly associated with treatment efficacy. Further, resistance alleles for C3435T were more common among Peuhls (19.57%) and the C allele of the treatment-sensitive phenotype was more common among Wolofs (16.25%).
Conclusion: TT genotypes of exon 12 and CC of exon 26 of MDR1 confer a high risk of developing resistance to anticancer drugs and are the predictive factors of the worst therapeutic result.
Keywords
Cancer; Breast; MDR1; Polymorphisms; Chemotherapy; Anthracyclines; Resistance
Introduction
Breast cancer is the most commonly diagnosed cancer in females (24.2%) and accounts for approximately a quarter of all new cancer cases worldwide. It was the most common cancer in 154 of 185 countries covered by GLOBOCAN in 2018 (WHO,2018). Cancer is associated with abnormal cell growth, which deregulates cell proliferation and death. This dysregulation may have several causes, such as heredity, action of a mutagenic compound, translocation of part of a chromosome, or any other mechanism that may lead to abnormal transcription or translocation of part of the DNA [1]. Several therapeutic models can restore this dysregulation, and reduce tumors or eliminate them definitively. Therefore, cancer treatment involves eliminating tumors and suppressing cancer cells. Different types of treatments, such as surgery, radiotherapy, hormone therapy, targeted therapies, and chemotherapy, can be used alone or in combination.
Chemotherapy is the most suitable and widely used approach for breast tumors because breast cancer comprises solid tumors [2]. However, chemotherapy sometimes involves multifactorial, intrinsic, or acquired Multidrug Resistance Gene Resistance (MDR1), which is a major clinical problem, in cancer treatment [2].
MDR1 is used in the treatment of various human diseases and is well known for its role in drug resistance. Hoffmeyer studied and described polymorphisms in this gene in a Caucasian population [3]. In this study, they reported a significant correlation between a polymorphism in exon 26 (C3435T) of MDR1 and the level of expression and function of P-glycoprotein (P-gp). Several studies have reported that polymorphisms in MDR1 can not only modify the affinity of the substrate for P-glycoprotein (P-gp), and consequently the pharmacokinetics of certain drugs used in cancer chemotherapy, but could also be involved in the mechanisms of chemoresistance [4]. In addition, previous studies have shown that cells that express MDR1 are resistant to a large number of cytotoxic agents such as taxanes, anthracyclines, vinca alkaloids, epipodophylotoxins, actinomycin D, mitomycin C and several other drugs [5]. Therefore, it is essential to have reliable predictive criteria for therapeutic responses to offer personalized treatment. This would make it possible to limit indications to individuals subjected to harmful treatments and identify those for whom the latter would be highly appropriate.
This work encouraged us to propose the hypothesize that polymorphisms in MDR1 are involved in the response and/or toxicity of anticancer agents in Senegalese females with breast cancer. For the first time, we aimed to determine the effect of MDR1 polymorphisms on the efficacy of anthracycline treatment for breast cancer in Senegalese females. Specifically, this will involve (i) determining the frequency distribution of Single Nucleotide Polymorphisms (SNPs) in MDR1, (ii) assessing responses to treatment and comparing the efficacy of the Fluorouracil-Adriamycin-Cyclophosphamide (FAC) and Doxorubicin-Cyclophosphamide (AC) protocols, (iii) assessing the association between C1236T and C3435T polymorphisms in MDR1 and the efficacy of anthracycline chemotherapy and (iv) determining the association between the C1236T and C3435T polymorphisms and ethnicity.
Materials and Methods
Study population and samples
This study was conducted among Senegalese females with breast cancer between March 2018 and August 2018 at the Joliot Curie Institute of the University Hospital of Aristide Le Dantec. Breast cancer cases are diagnosed via clinical assessment, mammography, or ultrasound and confirmed through anatomopathological examination of a cytological, histological, or surgical biopsy. Females who met the inclusion criteria (Senegalese origin, with a breast tumor larger than 2 cm, receiving neoadjuvant chemotherapy including anthracyclines) and signed an informed consent form were included in the study. They participated in the study without any direct individual benefit since they did not modify the therapeutic strategy of the patients included according to the results. As a result, each patient participated in the study from the beginning of the initial cancer evaluation to the assessment of clinical response using two distinct anthracycline protocols, FAC or AC. This study was approved by the Ethics and Research Committee of the Cheikh Anta Diop University, Dakar (protocol 0271/2018/CER/UCAD).
Choice of chemotherapy protocols
To improve the therapeutic approach and optimize treatment, a neoadjuvant chemotherapy model was selected. Patients received neoadjuvant chemotherapy with a concomitant model combining the same molecules according to the prescribed treatment protocol either FAC or AC.
Assessment of response after treatment
In this study, the assessment of post-treatment responses was based on clinical data. In terms of the clinical responses, we defined the following items:
- A complete Clinical Response (CR) was defined as the absence of a palpable tumor and detectable axillary adenopathy.
- A partial Clinical Response (PR) was defined as a 50% or greater reduction in tumor mass.
- Absence of response (S) was characterized by a tumor that retained its initial size.
- Progressive disease (P) was defined by an increase in tumour size despite treatment.
Patients with complete or partial response were considered responders, whereas those with stable or progressive disease were considered non-responders.
Genetic study
Total DNA was extracted from the blood using the standard Qiagen method (Qiagen DNeasy Blood Kit). Exons 12 and 26 of MDR1 were amplified using polymerase chain reaction using the following primers, (exon 12, 5′-TTTTTCTCACGGTCCTGGTAG-3′; 5′-CATCCCCTCTGTGGTCATA-3′); (exon 26, 5′-TTGATGGCAAAGAAATAAAGC-3′; 5′-CTTACATTAGGCAGTGACTCG-3′). Both exons were amplified under the following conditions, initial denaturation at 94°C for 5 min, 35 cycles (denaturation at 94°C for 30 s, hybridization at 45°C for 30 s and elongation at 72°C for 30 s) and final elongation at 72°C for 5 min. Amplifications were performed with 1 µL of DNA, 0.5 µL of dNTP, 1 µL of MgCl2, 0.2 µL of Taq polymerase, 0.25 µL of each primer, 2.5 µL of 10 × buffer, and 19.3 µL of water.
Genotyping was performed using Restriction Fragment Length Polymorphism (RFLP). Enzymatic digestion was performed using the PCR products incubated with a restriction endonuclease. In the presence of a mutation, digestion produces different fragments. The different genotype variants and their sizes are shown in Table 1. Electrophoretic migration was performed on a 3% agarose gel.
| Polymorphisms | Taille (pb) | Restriction enzymes | Genotype variants | RLFP conditions | ||
|---|---|---|---|---|---|---|
| CC (pb) | CT (pb) | TT (pb) | ||||
| Exon 26 (C3435T) | 207 | DpnII | 62 145 |
62 145 207 |
207 | 37°C for 1 h |
| Exon 12 (C1236T) | 147 | HaeIII | 85 62 |
147 85 62 |
147 | 37°C for 1 h |
Statistical analysis
The general characteristics of the patients were analyzed using standard descriptive statistics methods, that is, the calculation of frequencies and percentages for the categorical variables (ethnicity, histology, grade, stage, and number of treatments) and medians, extreme values, means, and standard deviations for the continuous variable age. The Shapiro-Wilk test was used to verify the normality of the age variables. A comparison of the mean values (using t- or Wilcoxon test) was used to determine the influence of age on response to treatment [6].
The different types of responses obtained at the end of the treatment, that is, RC, RP, S, or P, were classified into two groups. They are the responder group, comprising RC and RP and the non-responder group, comprising P and S.
The association between the SNPs (C1236T and C3435T) and the response after treatment with anthracyclines was evaluated using the chi-square test of independence and in cases where the numbers were <5, the Fisher's exact test was used. Correlation coefficients were calculated between two variables. The coefficient (phi) ranges from 0-1; between 0.8 and 1, the strength of association between the two variables is strong; between 0.5 and 0.8, it is moderate; between 0.2 and 0.5, it is weak; and below 0.2, it is extremely weak [6]. All analyses were performed using R studio version 3.5.1 (https://support.rstudio.com) and XLSAT version 7.55 (https://www.xlstat.com/en/).
For the Hardy–Weinberg (HW) equilibrium and linkage disequilibrium tests, we used Genepop software version 4.3 (https://kimura.univ-montp2.fr). For all the tests, the significance level was maintained at 5%.
Results
General characteristics of the population
Between March 2018 and August 2018, we collected data from 118 patients with breast cancer treated with Neoadjuvant chemotherapy at the Institute Joliot Curie, Hospital Aristide Le Dantec. Fifty-eight patients met the inclusion criteria. Of these, 27 received chemotherapy with FAC (46.55%) and 31 received chemotherapy with AC (53.45%). Twenty patients (34.4%) had distant metastases, with the most frequent sites being the lungs and bones.
In terms of medical history, three patients had hypertension and one had diabetes and hypertension. Our results also showed that 76.6% of the patients were not using contraception. Among 23.4% patients using contraception, 18.75% were on hormonal contraception, 6.24% were on injectable contraception and 31.25% were taking pills. A total of 53% of our patients were postmenopausal and 47% were still able to conceive children.
The age of the study population ranged from 25-70 years. The mean patient age was 42 ± 9 years. The incidence increases after the age of 30 years, peaking between the ages of 50 and 74 years. Ninety percent of the patients were aged >30 years. According to the FAC protocol, the age of patients varied between 25 and 61 years, with an average of 38 ± 8 years and a median of 40 years. The majority of patients were over 30 years old (85.18%). The age of the population treated with AC protocol ranged from 31-70 years, with an average age of 45 ± 10 years and a median age of 44 years. All patients in this population were over 30 years old. The normality test showed that the age distribution was not normal (p=0.031).
In terms of age, the individuals were homogeneously distributed according to the type of response to treatment and histological type of the tumor as shown in Figure 1. The results of the Wilcoxon test showed p-values >0.05, indicating that the differences in the type of response and histological type were not a function of age.
In this study, the nonspecific type of IDC (48.27%) was the most frequent, followed by ILC (29.32%) and IDCS (22.41%). The same trend was observed when the two protocols were analyzed separately. The differences between the two protocols were not statistically significant as shown in Table 2.
| Histology | Overall percentage (%) | Protocol (%) | p-value | |
| AC | FAC | 0.99 | ||
| IDC NOS | 48.27 | 25.86 | 22.41 | |
| IDCS | 22.41 | 12.07 | 10.34 | |
| ILC | 29.32 | 15.52 | 13.80 | |
According to the classic clinico-pathological characteristics (tumor size, lymph node invasion, and SBR grade (Scarff-Bloom and Richardson)), 10.35% of the patients had tumors of SBR grade I, 62.07% with SBR II, and 27.59% with SBR grade III. No significant differences were observed between the two groups as shown in Table 3.
| SBR | Overall percentage (%) | Protocol (%) | p-value | |
| AC | FAC | 0.12 | ||
| SBR I | 10.34 | 03.44 | 06.90 | |
| SBR II | 62.07 | 37.93 | 24.14 | |
| SBR III | 27.59 | 12.07 | 15.52 | |
The tumors were at stages IIA (1.8%), IIB (9.0%), IIIA (10.9%), IIIB (11.0%), IIIC (20.1%) and IV (47.2%). Most patients treated using the FAC protocol had SBR II (51.86%) or stage IV (58%) tumors. The same distribution was observed in patients treated using the AC protocol (70.96% with SBR grade II and 42% with stage IV tumors). No significant difference was observed in the distribution of these stages between the two protocols (p=0.44).
In this study, 7 patients received 3 courses of chemotherapy, 23 patients received 4 courses, 11 patients received 5 courses and 17 patients received 6 courses. Of the patients who underwent FAC, the majority (40.74%) underwent six cycles of chemotherapy. In contrast to the FAC protocol, the majority (48.38%) of patients received four courses of chemotherapy in the AC protocol. Significant differences were observed between the FAC and AC groups (p=0.0038) as shown in Table 4.
| Protocol | Number of courses of chemotherapy (%) | p-value | |||
| 3 cures | 4 cures | 5 cures | 6 cures | 0.0038 | |
| AC | 19.35 | 48.38 | 12.92 | 19.35 | |
| FAC | 3.70 | 29.64 | 25.92 | 40.74 | |
The overall population comprises seven different ethnic groups. Most were from Wolof and Peuhl. To obtain a better representation of the ethnic parameters, we divided the results into four groups, Wolofs (32.76%), Peuhls (24.14%), Sérères (18.97%), and another group (24.14%), comprising ethnic groups with low numbers (Diola, Sarakhoule, Bambara, Maure, and Soce). Our results showed that Sereres and Wolofs were the majority of patients treated with the FAC and AC protocols, respectively.
Response to treatment
After Neoadjuvant chemotherapy, clinical and ultrasound re-evaluations showed a frequency of CR of 8.62%, PR of 13.79%, S of 29.81% and P of 47.78%. In the general population (FAC and AC) and within the FAC population, the frequencies of non-responders were 56.90% and 55.56%, respectively. However, in the population receiving the AC protocol, the frequency of patients who were sensitive to treatment was higher than that of patients who were resistant to treatment (58.06% vs. 41.94%). The comparison between the two protocols (FAC and AC) showed an advantage in favor of the AC protocol. However, this difference was not statistically significant (p=0.95).
Molecular analysis results
HW equilibrium and linkage disequilibrium test: The results of the HW compliance test reveal that our study population deviates significantly from HW equilibrium, with a highly significant p-value. The linkage disequilibrium test between the two exons yielded a nonsignificant p-value (p=0.40576). Therefore, we hypothesized that the genotypes of exon 12 correlate with hose of exon 26.
Distribution of allelic and genotypic frequencies: Among the 58 patients, the genotypes of 49 and 54 patients were determined for exons 12 and 26, respectively. For exon 12, the frequencies of CC, CT, and TT genotypes were 28.57%, 14.28%, and 57.15% respectively. The allelic frequency of C was 35.71% and that of T was 64.29%. For exon 26, the frequencies of the CC, CT, and TT genotypes were 42.59%, 37.07% and 20.34% respectively. Our results revealed a predominance of the C allele (67.18%) over the T allele (32.82 %). These differences were statistically significant between the two exons as shown in Table 5.
| Exons | SNP | Genotypes | Frequencies | P-value | Alleles | Frequencies | P-value |
| 12 | C1236T | C/C | 28.57% | 0.001* | C T | 35.71% 64.29% | 0.000* |
| C/T | 14.28% | ||||||
| T/T | 57.15% | ||||||
| 26 | C3435T | C/C | 42.59% | C T | 67.18% 32.82% | ||
| C/T | 37.07% | ||||||
| T/T | 20.34% |
Note: *Significant difference; C, T are the Alleles frequencies.
Distribution of exon 12 allelic and genotypic frequencies according to protocol type: A higher prevalence of the T allele (83.34%) than the C allele (16.66%) was observed in the population treated with FAC. The frequencies of the CC (14.28%) and CT (4.76%) genotypes were lower than that of the homozygous TT genotype (80.96%). In the AC population, the frequencies recorded showed that the CC homozygote was more frequent (39.28%), followed by the CT heterozygote (21.14%) and then the TT homozygote (39.58%). The T allele (50.15%) dominated over the C allele (49.85%). A comparison of allelic and genotypic frequencies between populations (FAC vs. AC) revealed significant differences as shown in Table 6.
| Exon | Protocol | Genotypes | p-value | Alleles | p-value | |||
| C/C | C/T | T/T | 2.11e-7 | C | T | 0.00 | ||
| 12 | FAC | 14.28% | 04.76% | 80.96% | 16.66% | 83.34% | ||
| AC | 39.28% | 21.14% | 39.58% | 49.85% | 50.15% | |||
Distribution of allelic and genotypic frequencies of exon 26 according to protocol type: Equal frequencies (37.04%) were observed for CC homozygotes and CT heterozygotes in the population treated with FAC. In this population, the frequency of TT genotype was 25.94%. The C allele, which covers 55.56% of the population, was more frequent than the T allele, with a frequency of 44.44%. The results obtained in the population treated with the AC protocol showed that the C allele was present in the majority (66.65%), followed by the T allele (33.35%). The CC homozygote was the most represented (48.14%), followed by the CT heterozygote and TT homozygote, with frequencies of 37.03% and 14.83%, respectively.
A comparison of the allelic and genotypic frequencies between the populations treated with FAC and those treated with AC showed non-significant differences as shown in Table 7.
| Exon | Protocol | Genotypes | p-value | Alleles | p-value | |||
| C/C | C/T | T/T | 0.10 | C | T | 0.17 | ||
| 26 | FAC | 37.03% | 37.03% | 25.94% | 55.56% | 44.44% | ||
| AC | 48.14% | 37.03% | 14.83% | 66.65% | 33.35% | |||
Results of association analyses
Correlation between C1236T genotypes and treatment efficacy: The analysis showed a significant association between responding patients and the CT genotype, whereas patients with the TT genotype were associated with resistance to chemotherapy, with a low association coefficient (p=0.049, phi=0.18).
In the population treated with the FAC protocol, we observed a significant association between SNP C1236T and the response to chemotherapy (p=0.020, phi=0.10). The contributory parts of this correlation showed that patients carrying CC and CT genotypes were more sensitive to anthracycline treatment FAC. In contrast, carriers of the TT genotype were resistant to the treatment as shown in Figure 2.
In the population treated with the AC protocol, no correlation was observed between SNP C1236T and the type of response after treatment (p=0.99).
Correlation between C3435T genotypes and treatment efficacy: Our results showed a significant association between the CC genotype and treatment resistance. In addition, patients carrying the TT and CT genotypes are pharmacosensitive.
In the population treated using the FAC protocol, Fisher's exact test showed a significant association between the CC genotype and treatment resistance. Our results also showed that patients who responded to treatment were significantly correlated with the TT and CT genotypes (p=0.009 and phi=0.41, respectively).
The results of the correlation test showed a significant association between the population treated with the AC protocol and the different genotypes observed (p=0.003), with a Pearson’s phi coefficient of 0.39. Fisher's exact test showed that TT and CT genotypes were correlated with pharmacosensitivity. This is in contrast to the CC genotype, which was correlated with drug resistance as shown in Figure 3.
Correlation between C1236T genotypes and ethnicity: We found a significant relationship between the C1236T genotypic profiles and ethnic group (p=0.002 and phi=0.45). Fisher's exact test showed a weak but significant association between the CC genotype and the Wolof and Peuhl ethnic groups and a significant association between the TT and CT genotypes and the Peuhl and Serer ethnic groups as shown in Figure 4.
Correlation between C3435T genotypes and ethnicity: There was a highly significant positive correlation between the C3435T genotypes and different ethnic groups (p=0.010 and phi=0.40). Fisher’s exact test showed a weak but significant association between the genotypes (CT and TT) and the Wolof ethnic group, and a significant relationship between the CC genotype and the Peuhl ethnic group as shown in Figure 5.
Discussion
The C1236T and C3435T polymorphisms in exons 12 and 26 of MDR1 have been shown to correlate with P-glycoprotein function and may influence Interindividual variability in the bioavailability and pharmacokinetics of various drugs [7,8]. It is an active transmembrane pump that transports numerous drugs. We studied the frequency of these SNPs and their role in predicting response to Neoadjuvant anthracycline-based chemotherapy for breast cancer in Senegalese females.
The results concerning clinico-pathological parameters showed heterogeneity in populations. Age predicts the response to chemotherapy. More than 80% of our study population was over 30 years old, supporting the WHO (2017) report that many low- and middle-income countries face the burden of breast cancer, which is extremely lethal for females over 30 years of age. Among the patients, 84.4% were under 50 years of age and 46.55% were between 40 and 50 years of age. Mawadzoue states that breast cancer in African females is characterized by a predominance of pre-menopausal cancers, with more than 70% of cases affecting females under 50 years of age, and peak incidence in the 39-49 age groups [9]. A diverse ethnic distribution was observed in our population, over 60% of who were from Wolof and Peuhl ethnic groups. In addition, 40.74% of the responding patients received six cycles of chemotherapy. We characterize breast the different types of breast cancer based on histology. In this study, the histological types IDC NOS and IDCS were the most frequent types. This is in line with the study by Diane, which reported that breast cancers in situ, which represent 20% of all breast cancers, consist of lobular carcinoma in situ in 10%-15% of cases and Ductal Carcinoma in Situ (IDCS) in a larger majority (80%-85%). Non-specific IBC is thought to account for 60%-75% of breast cancers.
Our study population did not follow HW equilibrium, which could be due to frequent exposure to chemotherapy products, thus increasing the expression of P-gp, which may be an evolutionary force pushing the population towards HW disequilibrium. A linkage disequilibrium test showed that the two exons were not independent (p=0.40576). Some researchers have suggested that certain polymorphic sites (C1236T in exon 12, C3435T in exon 26, and G2677T/A in exon 21) of MDR1 are linked and may modify P-gp activity and function [10-13]. Regarding the C1236T polymorphism in exon 12, the frequency distribution of genotypes within the study population showed a higher prevalence of the TT homozygote (57.15%), followed by CC (28.57%) and CT (14.28%). The T allele was more prevalent in the study population (64.29%) than the C allele (35.71%). Ryu found in their study that the TT homozygote of SNP C1236T was more prevalent in Caucasians and Asians [14]. For exon 26, we noted a significantly higher prevalence of the CC homozygote (42.59%) and the C allele (67.18%) in the study population. As shown by Balram, the frequency of the wild-type C allele is higher in Africans (73%-83%) than in Asians (38%-48%) and Caucasians (52%) [15].The frequency of the CC genotype is higher in African populations and lower in Southwest Asian populations [12]. According to fung and in line with our findings, C alleles are more frequent than T alleles and are therefore considered to be wild-type alleles. Africans have a significantly higher frequency of wild-type alleles (>74%) and at least 50% individuals carry both C alleles [16].
Our results also showed that the proportion of patients who were resistant (56.90%) to treatment (FAC and AC) was higher than that of susceptible patients (43.10%). This result may have concealed some information. Under the FAC protocol, we observed a higher rate of resistance to treatment (55.56%) than the response rate (44.44%). This contrasts with the results observed in the population treated with the AC protocol, in which 58.06% responded favorably to the treatment. However, these differences were not significant as the results yielded a p-value equal to 0.95. The comparison between the FAC and AC protocols showed a difference in efficacy that was more favorable for the AC protocol, in accordance with the study by Roché [17]. This is in line with a study by Frénel and Campone, which showed that anthracycline-based regimens FAC, 5-fluorouracil, Epirubicin and Cyclophosphamide (FEC) and AC reduced the risk of relapse by 11.2% (p<0.00001) [18].
This low response rate can be attributed to several factors. However, socioeconomic aspects must also be considered. Chemotherapy is an expensive treatment, and access to it remains a challenge for some patients. At Hospital Aristide le Dantec, the average cost of chemotherapy treatment was 195,000 CFA francs per session. There is a three-week gap between the courses of chemotherapy, and a complete blood count must be performed before each course to check for neutropenia. In addition, patients must be administered various other supportive medications (such as antinausea drugs, antibiotics) to help them cope with the side effects of cancer treatments. Therefore, these patients are often absent or late for chemotherapy appointments. This could have drastic consequences for treatment outcomes.
We observed no significant relationship between the C1236T polymorphism of exon 12 and the response to chemotherapy combined with the FAC and AC protocols (p=0.1933) for breast cancer in Senegalese females. However, in our study population, we noted a predominance of the T allele (68.33%) in non-responders compared to responders (58.89%). CC homozygotes (31.57%) and CT homozygotes (21.05%) were dominant in responders, whereas TT homozygotes (63.33%) were dominant in anthracycline-resistant patients. Kim showed that mutations at position 1236 of exon 12 of MDR1 confer drug resistance in several different diseases [19]. Similarly, Chaturvedi and Zhou found a significant association between the SNP C1236T and response to treatment (p=0.018) [20,21].
A borderline significant correlation was found between the response to chemotherapy and SNP C3435T in exon 26 of MDR1 (p=0.05). This result is consistent with that reported by Rodrigues in patients with breast cancer in Brazil [22]. Our analysis revealed a significant prevalence of the T allele and CT homozygotes (45.84%) of the C3435T SNP in patients who had a CR. This is in agreement with the study by Kafka, which revealed that the TT genotype at position 3435 was significantly correlated (p=0.029) with a CR to chemotherapy with anthracyclines alone or in combination with taxanes [23]. The association between SNP and MDR1 expression was first reported by Hoffmeyer. In their study, the presence of the homozygous T allele resulted in reduced MDR1 expression, and the 3435T allele was associated with a two-fold reduction in MDR1 expression in the duodenum [3].
We observed a significant correlation between ethnic groups and chemotherapy response to anthracyclines (p=0.013). As mentioned earlier, for exon 12, the T allele carries a resistant phenotype. The results concerning the correlation between ethnicity and the C1236T polymorphism revealed a significant relationship between the Wolof ethnic group and the CT genotype. In contrast, Serer and Peuhl ethnic groups showed higher frequencies of the T allele. Based on these results, we can deduce that there is a significant correlation between chemotherapeutic sensitivity and ethnicity. This association is linked to the association between ethnic groups and patient genotypic profiles.
With regard to exon 26, the proportion of CC homozygotes of the SNP C3435T was higher in Peuhls. We also noted that the CT heterozygote was more prevalent in the Wolof ethnic group than in the other ethnic groups. The association study revealed a significant correlation between chemotherapeutic sensitivity and the Wolof ethnic group and a significant correlation between the Peuhl- and Serer-resistant phenotypes. According to Kim, patients with the TT genotype of the SNP C3435T have high P-gp activity and, consequently, higher levels of MDR1 expression, offering greater protection against the accumulation of active substances in anticancer agents [24,25]. Other researchers have speculated that silent variation in SNP C3435T could reduce translation efficiency and influence functional consequences [8].
This study showed that the Wolof ethnic group had a better response to chemotherapy than other ethnic groups. In contrast, the Peuhl and Serere ethnic groups showed greater resistance to anthracycline treatment combined with the FAC and AC protocols than other ethnic groups.
Conclusion
We observed that the C1236T and C3435T polymorphisms in exons 12 and 26 of MDR1 had a considerable impact on the efficacy of Neoadjuvant anthracycline-based breast cancer chemotherapy. Consequently, the TT genotype in exon 12 and CC genotype in exon 26 of MDR1 are risk factors for resistance to anticancer drugs. In the future, our results suggest the inclusion of genotypic profiling of MDR1 in the pre-chemotherapy work-up and guided drug selection based on the patient's genotype.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127(12):2893-2917.
[Crossref] [Google Scholar] [PubMed]
- Gligorov J. Polymorphisms and neoadjuvant treatments of breast cancer: Effectiveness of docetaxel and ABCB1/MDR1 polymorphism. 2012;183p.
- Hoffmeyer SO, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, et al. Functional polymorphisms of the human multidrug-resistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 2000;97(7):3473-3478.
[Crossref] [Google Scholar] [PubMed]
- Sauna ZE, Smith MM, Muller M, Ambudkar SV. Evidence for the vectorial nature of drug (Substrate)-stimulated ATP hydrolysis by human p-glycoprotein* 210. J Biol Chem 2001;276(36):33301-33304.
- Holland IB, Cole SP, Kuchler K, Higgins CF. ABC proteins: From bacteria to man. 2003; p35-391.
- Huguier M, Flahault A. Biostatistiques au quotidien. 2ND edn, Dectire, 2003.
- Kurata Y, Ieiri I, Kimura M, Morita T, Irie S, Urae A, et al. Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of Pâ?glycoprotein. Clin Pharmacol Ther 2002;72(2):209-219.
[Crossref] [Google Scholar] [PubMed]
- Schwab M, Eichelbaum M, Fromm MF. Genetic polymorphisms of the human MDR1 drug transporter. Annu Rev Pharmacol Toxicol 2003;43(1):285-307.
[Crossref] [Google Scholar] [PubMed]
- Mawadzoue FD. Breast (female) and liver cancers in West Africa: Temporal evolution of incidence and assessment of risk factors in Gambia and Mali. 2011; 170p.
- Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther 2001;70(2):189-199.
- Tanabe M, Ieiri I, Nagata N, Inoue K, Ito S, Kanamori Y, et al. Expression of P-glycoprotein in human placenta: Relation to genetic polymorphism of the Multidrug Resistance (MDR)-1 gene. J Pharmacol Exp Ther 2001;297(3):1137-1143.
- Asano T, Takahashi KA, Fujioka M, Inoue S, Okamoto M, Sugioka N, et al. ABCB1 C3435T and G2677T/A polymorphism decreased the risk for steroid-induced osteonecrosis of the femoral head after kidney transplantation. Pharmacogenetics 2003;13(11):675-682.
- Anglicheau D, Laurent-Puig P, Becquemont L, Cassinat B, Beaune P, Legendre C, et al. Association of the MDR-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol 2003;14(7):1889-1896.
- Ryu HC, Kwon HY, Choi IK, Rhee DK. Analyses of single nucleotide polymorphisms and haplotype linkage of the human ABCB1 (MDR1) gene in Korean. Arch Pharm Res 2006;29(12):1132-1139.
- Balram C, Sharma A, Sivathasan C, Lee EJ. Frequency of C3435T single nucleotide MDR1 genetic polymorphism in an Asian population: Phenotypic–genotypic correlates. Br J Clin Pharmacol 2003;56(1):78-83.
- Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim Biophys Acta 2009;1794(5):860-871.
- Roché H, Fumoleau P, Spielmann M, Canon JL, Delozier T, Serin D, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: The FNCLCC PACS 01 Trial. J Clin Oncol 2006;24(36):5664-5671.
- Frénel JS, Campone M. Chemotherapy of non-metastatic breast cancers: State of play in 2010. J Gynecol Obstet Biol Reprod 2010;39(8):F79-84.
- Kim IW, Peng XH, Sauna ZE, FitzGerald PC, Xia D, Müller M, et al. The conserved tyrosine residues 401 and 1044 in ATP sites of human P-glycoprotein are critical for ATP binding and hydrolysis: Evidence for a conserved subdomain, the A-loop in the ATP-binding cassette. Biochemistry 2006;45(24):7605-7616.
- Chaturvedi P, Tulsyan S, Agarwal G, Lal P, Agarwal S, Mittal RD, et al. Influence of ABCB1 genetic variants in breast cancer treatment outcomes. Cancer Epidemiol 2013;37(5):754-761.
- Zhou Z, Chen Q, Zuo D, Wang H, Hua Y, Cai Z. ABCB1 (rs1128503) polymorphism and response to chemotherapy in patients with malignant tumors-evidences from a meta-analysis. Int J Clin Exp Med 2015;8(1):265-272.
- Rodrigues FF, Santos RE, Melo MB, Silva MA, Oliveira AL, Rozenowicz RL, et al. Correlation of polymorphism C3435T of the MDR-1 gene and the response of primary chemotherapy in women with locally advanced breast cancer. Genet Mol Res 2008;7(1):177-183.
- Kafka A, Sauer G, Jaeger C, Grundmann R, Kreienberg R, Zeillinger R, et al. Polymorphism C3435T of the MDR-1 gene predicts response to preoperative chemotherapy in locally advanced breast cancer. Int J Oncol 2003;22(5):1117-1121.
- Kim HJ, Hwang SY, Kim JH, Park HJ, Lee SG, Lee SW, et al. Association between genetic polymorphism of multidrug resistance 1 gene and sasang constitutions. Evid Based Complement Alternat Med 2009;6:73-80.
- Leonessa F, Clarke R. ATP binding cassette transporters and drug resistance in breast cancer. Endocr Relat Cancer 2003;10(1):43-73.