Review Article - Journal of Molecular Pathophysiology (2024)
A Novel Intervention for Long COVID: The Combination of Dopamine D1 Partial Agonists and Candesartan
Adonis Sfera1*, Jacob Anton1 and Sabine Hazan22ProgenaBiome, Ventura Clinical Trials, California, USA
Adonis Sfera, Department of Psychiatry, Patton SAdonistate Hospital, California, USA, Email: dr.sfera@gmail.com
Received: 01-Sep-2024, Manuscript No. JMOLPAT-24-147083; Editor assigned: 03-Sep-2024, Pre QC No. JMOLPAT-24-147083 (PQ); Reviewed: 18-Sep-2024, QC No. JMOLPAT-24-147083; Revised: 25-Sep-2024, Manuscript No. JMOLPAT-24-147083 (R); Published: 02-Oct-2024
Abstract
Like previous pandemics, COVID-19 has been succeeded by well-documented postinfectious sequelae, colloquially known as “long COVID”. This syndrome, manifested by chronic fatigue, post-exertional malaise, shortness of breath, myalgia, and concentration difficulties, may last for many months after the acute phase of illness. Long COVID affects all aspects of a patient’s life, including work, rest, and activities of daily living, placing a large financial burden not only on the individual but also on families, and the society at large.
Like other viruses, including Human Immunodeficiency Virus (HIV), Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) may thrive in viral reservoirs, likely comprised of senescent endothelial cells, macrophages, and microglia that shield the virus from neutralizing antibodies, maintaining a low-grade SARS-CoV-2 infection. Since senescent cells upregulate Angiotensin-Converting Enzyme 2 (ACE- 2), the SARS-CoV-2 entry portal, this protein facilitates viral ingress in host cells.
The invention described here consists of a combination of dopamine D1 receptor antagonists or partial agonists, such as SKF 38393, and angiotensin receptor blocker, candesartan, for long COVID. This strategy is based on the hypothesis that the SARS-CoV-2 virus thrives in senescent endothelial cells, macrophages, and microglia and can be reactivated under favorable circumstances. Furthermore, as both angiotensin II and dopamine D1 receptors have been implicated in cellular senescence, manipulating these proteins may avert viral persistence in reservoirs and the residual COVID-19 symptoms.
Keywords
Chronic pain; Brain fog; Sartans; Dopamine; Receptors; Angiotensin II
Introduction
The SARS-CoV-2 virus exploits cellular senescence, a program adopted by cells to defend against malignant transformation. The main characteristics of cellular senescence include proliferation arrest, resistance to apoptosis, active metabolism, and generation of a toxic secretome, known as Senescence Associated Secretory Phenotype (SASP). SASP can induce senescence in the surrounding healthy cells by exocrine/paracrine route. Due to their continuous contact with the blood, senescent Endothelial Cells (ECs) likely release SASP directly into the systemic circulation, promoting organismal aging. Many viruses, including SARS-CoV-2, thrive in senescent cells because of elevated iron and calcium (Ca2+) content that benefit viral progeny. Moreover, the mammalian Target of Rapamycin (mTOR) activation in senescent cells leads to repression of autophagy, preventing the elimination of aged or virus-infected cells (a process referred to as efferocytosis). Furthermore, senescent macrophages and their Central Nervous System (CNS) counterparts, microglia, disrupt host antiviral defenses, further generating a virus-hospitable environment.
Literature Review
The SARS-CoV-2 virus can induce cellular senescence directly or indirectly by activating Human Endogenous Retro Viruses (HERVs), ancient viral elements comprising about 8% of the human genome.
Other fatiguing illnesses, including Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/ CSF), Gulf War Syndrome (GWS), and Cancer-Related Fatigue (CRF) have been associated with premature cellular senescence.
At the level of gut barrier, senescent ECs, and Intestinal Epithelial Cells (IECs) disrupt tight junctions, the molecular Velcro that holds cells together, increasing permeability. Enlarged intercellular spaces facilitate microbial migration outside of the Gastro Intestinal (GI) tract [1]. Intestinal microorganisms are tolerated in the gut lumen but can trigger inflammation and immunogenicity once translocated into the systemic circulation.
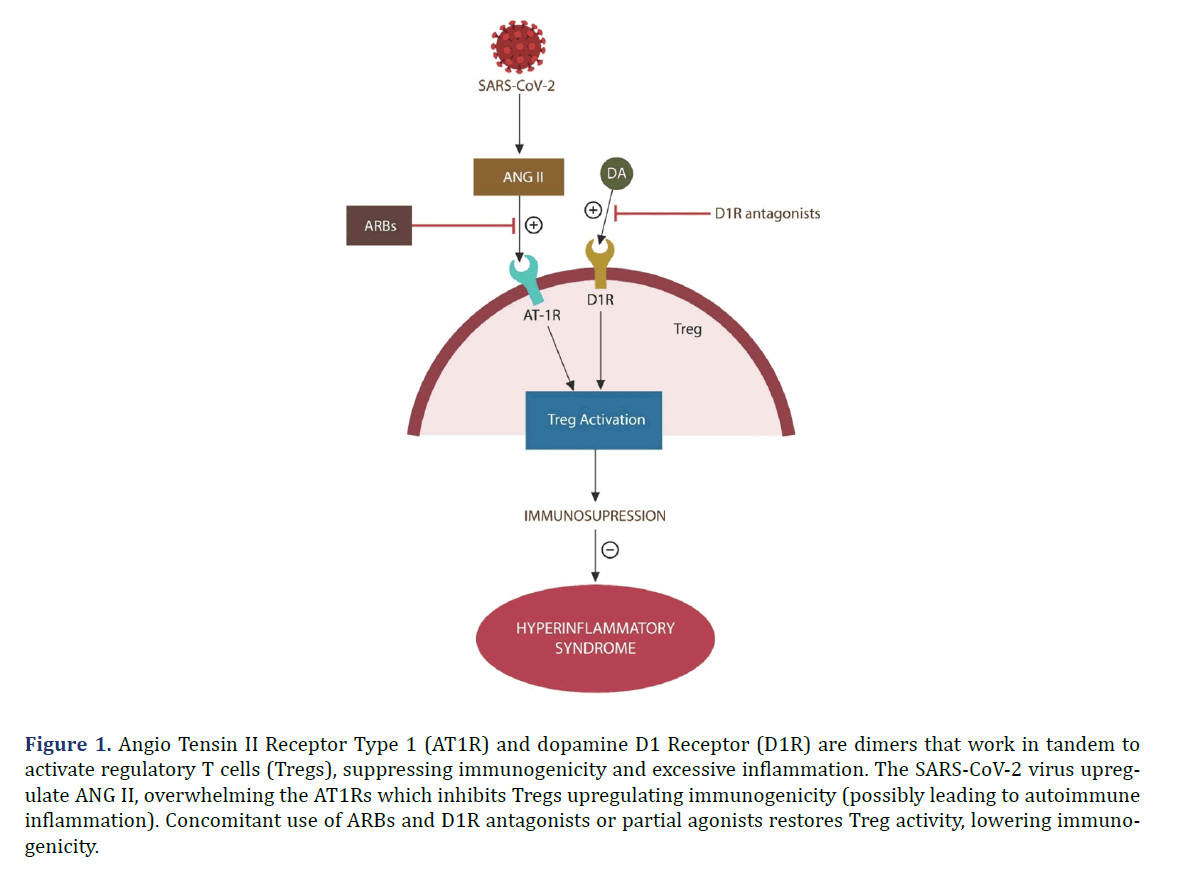
Regulatory T cells (Tregs) are important for maintaining GI tolerance to food and gut commensal microorganisms. Tregs express both Angiotensin II Type 1 Receptors (AT1Rs) and Dopamine D1 Receptors (D1Rs) which function as dimers via receptor-receptor interaction [2] as shown in Figure 1.
Figure 1. Angio Tensin II Receptor Type 1 (AT1R) and dopamine D1 Receptor (D1R) are dimers that work in tandem to activate regulatory T cells (Tregs), suppressing immunogenicity and excessive inflammation. The SARS-CoV-2 virus upregulate ANG II, overwhelming the AT1Rs which inhibits Tregs upregulating immunogenicity (possibly leading to autoimmune inflammation). Concomitant use of ARBs and D1R antagonists or partial agonists restores Treg activity, lowering immunogenicity.
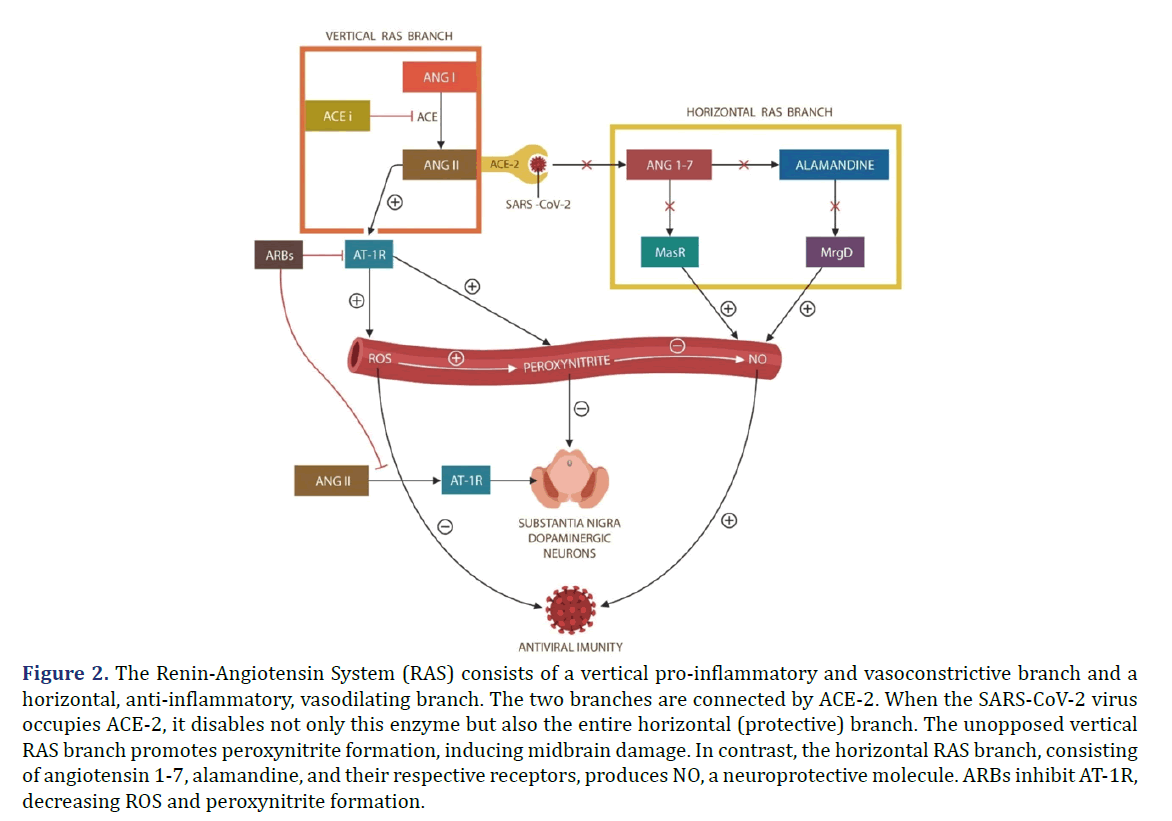
SARS-CoV-2 infection upregulates Angiotensin II (ANG II), a mitochondrial toxin, which under normal circumstances is promptly hydrolyzed by Angiotensin- Converting Enzyme 2 (ACE-2). However, since ACE-2 is the SARS-CoV-2 entry portal, it is disabled by viral attachment, leading to unopposed accumulation of ANG II as shown in Figure 2. In addition, since ACE-2 connects the vertical branch of the Renin- Angiotensin System (RAS) with the protective horizontal RAS branch, SARS-CoV-2-disabled ACE-2, removes the beneficial effects of the horizontal RAS branch. Consequently, Reactive Oxygen Species (ROS), especially peroxynitrite, generates cardiovascular and Central Nervous System (CNS) pathology, including Parkinson’s disease. As D1Rs are expressed in the midbrain and forebrain, they regulate motor behavior, and cognition, probably accounting for the “brain fog” experienced in long COVID [3]. Interestingly, fatigue was associated with midbrain deactivation, suggesting that long COVID exhaustion is likely caused by ROS damage [4]. Indeed, peroxynitrite was implicated in other fatiguing illnesses, such as multiple chemical sensitivity and ME/CFS [5,6].
Figure 2. The Renin-Angiotensin System (RAS) consists of a vertical pro-inflammatory and vasoconstrictive branch and a horizontal, anti-inflammatory, vasodilating branch. The two branches are connected by ACE-2. When the SARS-CoV-2 virus occupies ACE-2, it disables not only this enzyme but also the entire horizontal (protective) branch. The unopposed vertical RAS branch promotes peroxynitrite formation, inducing midbrain damage. In contrast, the horizontal RAS branch, consisting of angiotensin 1-7, alamandine, and their respective receptors, produces NO, a neuroprotective molecule. ARBs inhibit AT-1R, decreasing ROS and peroxynitrite formation.
For the above reasons, we propose a combination of a D1R partial agonist, SKF38393, and a Blood- Brain Barrier (BBB)-crossing Angiotensin Receptor Blocker (ARB), candesartan. Together, these agents likely restore the physiological function of Tregs, clear virus-infected cells, and avert cell-cell Fusion-Induced Senescence (FIS). These functions deny these cells the reservoir status, ameliorating the symptoms of long COVID as shown in Figure 2 [7-10].
Cell-cell fusion or syncytia formation
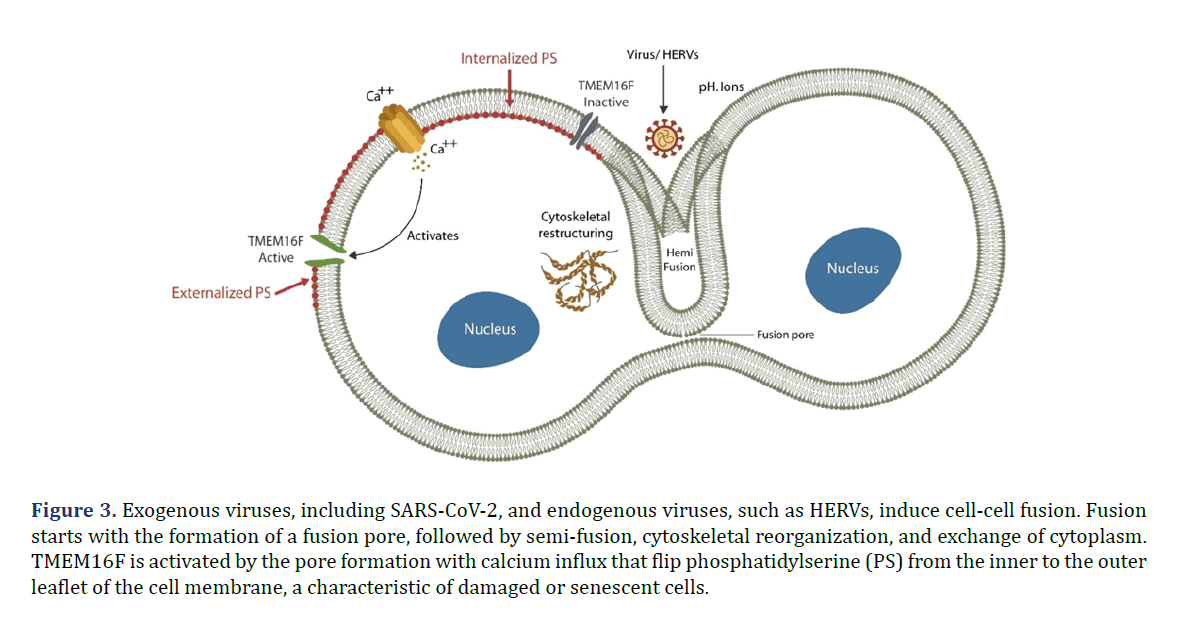
Exogenous or endogenous viruses can drive cell-cell fusion or syncytia formation, generating giant multinucleated cells. Fusion starts with a virus-induced fusion pore in the cell membrane that triggers Ca2+ influx, activating TMEM16F, a scramblase that promotes Phosphatidylserine (PS) externalization, marking the cell ready for apoptosis or fusion. Fused cells trigger premature cellular senescence, known as Fusion-Induced Senescence (FIS) [11]. These syncytial structures are resistant to elimination therefore, comprising ideal viral reservoirs capable of harboring the virus for a longer time [12].
SARS-CoV-2 upregulate ANG II, disrupting efferocytosis, the physiological mechanism of clearing damaged or senescent cells [13]. This in return alters the permeability of gut barrier and BBB, allowing microbial translocation outside of the GI tract. Translocated microbes and/or their components trigger further senescence, as tissues defend themselves against inflammation- mediated malignant transformation.
Fatiguing illnesses, including ME/CSF, GWS, CFS, and long COVID have been associated with premature cellular senescence and microbial translocation. Both candesartan and SKF38393 inhibit ANG II effects, restoring Tregs, physiological efferocytosis, and barrier permeability.
Selective Dopamine 1 Receptor (D1R) partial agonists
Several D1R partial agonists, such as SKF38393, may be useful in long COVID, including SKF75670, SKF77434, SKF83959, and SKF82957 PF8294, PF6142, PF-06649751 and others [14,15].
D1R is the most abundant dopamine receptor in both the rodent and human brain and its dysfunction was associated with many pathologies [16]. Several selective D1R partial agonists have been developed for the treatment of Parkinson’s disease, stimulant use disorders, and cognitive disorders [17-19]. Compared to newer agents, SKF38393, is a prototypical D1R selective partial agonist on which a treasure trove of data has been gathered by numerous studies. Newer D1R partial agonists may have similar effects. However, their action on immune and senescent cells is poorly defined at this time.
In preclinical studies, SKF38393 was well tolerated, and adverse effects were not very different than those of levodopa. A related, newer D1R partial agonist, PF- 06649751 which is under active investigation for Parkinson’s disease, was tested on human subjects and is currently in Phase I clinical trials (NCT02262767), (NCT02847650), (NCT01981694) [20].
Angiotensin Receptor Blockers (ARB)
Candesartan may be a preferred ARB in this invention due its greater potency and selectivity compared to other approved ARB’s as well as its ability to cross the Blood Brain Barrier (BBB) [21]. Telmisartan similarly crosses the BBB, while other approved ARB’s, including olmesartan, eprosartan, irbesartan, and losartan do not.
Candesartan is a synthetic, benzimidazole-derived ANG II receptor antagonist, a prodrug with numerous properties, including antihypertensive activity. Candesartan selectively competes with ANG II for the binding of AT1Rs in vascular smooth muscle, blocking ANG II-mediated vasoconstriction. In addition, antagonism of AT1R in the adrenal gland inhibits ANG II-stimulated aldosterone synthesis and secretion by the adrenal cortex. As a result, sodium, and water excretion increase, followed by a reduction in plasma volume and blood pressure.
The consequences of Virus-Induced Senescence (VIS)
The SARS-CoV-2 virus induces premature cellular senescence and thrives in senescent cells that likely become viral reservoirs, accounting for the symptoms of long COVID [22].
VIS is an infection-mediated pathology driven by two mechanisms. They are (1) merging host cells into multinucleate syncytia that trigger a phenomenon known as Fusion-Induced Senescence (FIS); and/or (2) upregulating cortisol and the High-Mobility Group Box 1 (HMGB1), molecules which disrupt efferocytosis, causing accumulation of senescent, virus-infected cells.
In the gut, senescent IECs and ECs increase permeability, enabling translocation of microbes and/or their components into the systemic circulation. This in turn promotes further senescence, engendering a vicious circle. For example, cellular senescence induced by Lipo Poly Saccharide (LPS), a gram-negative microbial molecule, has been well-documented [23,24].
In contrast, candesartan and SKF38393 (1) ameliorate the effects of premature cellular senescence; (2) enhance efferocytosis; (3) decrease the permeability of gut barrier to bacteria and/or their components.
This hypothesis is supported by the following data:
Several studies have documented that the SARS-CoV-2 virus, akin to its viral counterparts, has the capability to promote VIS, thereby creating a microenvironment suitable for the replication and proliferation of viral progeny [25,26]. This phenomenon underscores the interplay between viral infection and cellular aging [27]. Understanding these mechanisms is of paramount importance in devising effective therapeutic interventions and preventive strategies aimed at mitigating the impact of viral infections on human health [22]. For example, FIS has clarified the link between viral infection and cellular senescence [28,29].
Indeed, previous studies have associated translocated intestinal microbes with ME/CFS, an entity marked by cellular senescence and a clinical picture similar to that of long COVID [30-32]. In addition, CRF, a related syndrome associated with chemotherapy or radiation-induced cellular senescence, exhibits symptoms indistinguishable from those of long COVID [33-35]. Moreover, candesartan exerts anticancer properties by inhibiting AT1R, a receptor implicated in tumor vascularization and angiogenesis [36,37]. Candesartan is the most potent and selective of the currently available ARBs, and its action on ECs suggests antimetastatic potential, by withholding outgrowth vascularization [38].
Dopamine D1R partial agonists, like SKF38393, exerts anticancer activity and via its dimer AT-1R dysfunction triggers fatigue [39-41]. Indeed, ME/CFS, GWS, and long COVID have been linked to the unchecked accumulation of senescent cells due to dysfunctional macrophages/ microglia and NKCs, the key efferocytosis executors [42-44].
Both psychological and biological stressors are known to increase blood cortisol and HMGB1, biomolecules which directly impair efferocytosis [45-46]. HMGB1 and cortisol have been associated with Inflammatory Bowel Disease (IBD), a condition marked by significant fatigue, and dysfunctional gut barrier with microbial translocation [47]. Previous studies have connected the accumulation of senescent cells to inflammation and disruption of gut barrier and BBB, allowing microbes to migrate from the GI tract into host tissues and organs, including the brain [48,49].
NKCs and macrophage/microglia express viable dopaminergic and RAS systems, suggesting that candesartan and SKF38393 may directly benefit efferocytosis [50,51]. In addition, previous studies have shown that SKF38393 augments NKC cytotoxicity, while candesartan enhances microglial phagocytosis, facilitating the elimination of senescent cells, further emphasizing a direct potential effect on long COVID [52,53].
Viral reservoirs
Viral reservoirs are cellular phenotypes and tissue sites in which viruses can accumulate, replicate, and thrive after the acute phase of COVID-19. For example, microglia and memory CD4+ T cells are well-known HIV reservoirs in which the pathogen can persist in a latent state, averting exposure to the Highly Active Antiretroviral Therapy (HAART) [54]. Other known viruses that can maintain latency in humans are Herpes Simplex Virus (HSV), Varicella Zoster Virus (VZV), and Epstein Barr virus (EBV). For example, VZV can thrive in sensory- nerve ganglia, reservoirs from which it can be reactivated later in life [55].
It is hypothesized here that the SARS-CoV-2 virus thrives in senescent cells in a latent state. Senescent cells are metabolically active and long-lived as they resist apoptosis as well as other modalities of programmed cell death, including ferroptosis [56]. In addition, senescent cells upregulate iron, a biometal necessary for viral replication as well as Ca2+ which plays a key role in virion formation, and the translation of viral proteins [56]. Senescent macrophages, likely used by SARS-CoV-2 as reservoirs, upregulate fibrogenesis, facilitating fibrosis, a key pathology promoted by this virus [57]. Moreover, SARS-CoV2 has been shown to directly infect microglia, the CNS macrophages, inducing dystrophy or senescence, that may convert these cells into neurotoxic phenotypes, explaining the neuropsychiatric sequelae of long COVID, such as “brain fog” [58].
Taken together, the SARS-CoV-2 virus promotes premature cellular, including macrophage/microglia, senescence, and thrives in a latent state in these cells, inducing low-grade inflammation, a likely driver of long COVID.
Discussion
Cellular senescence and biological barriers
Cellular senescence is a physiological program that protects cells against malignant transformation by arresting replication in response to exogenous or endogenous insults [27]. Senescent cells are metabolically active and release SASP which can induce senescence in neighboring healthy cells, spreading both senescence and viral infection. Senescent cells upregulate intracellular iron and Ca2+, generating an ideal milieu for intracellular pathogens, including the SARS-CoV-2 virus [59,60].
For this reason, the virus not only promotes cellular senescence but may also “hide” in senescent cells, evading the neutralizing antibodies [60]. In addition, senescent cells exhibit shorter telomeres and overexpress ACE-2, the SARS-CoV-2 entry portal, facilitating viral ingress, likely explaining the higher risk of COVID-19 complications in older individuals [61-63]. This also explains the reason senolytic agents are believed beneficial in both acute COVID-19 and long COVID [64,65]. Although SKF38393 and candesartan are not considered senolytic drugs, they were shown to ameliorate the effects of ECs senescence [66].
COVID-19 and Fusion-Induced Senescence (FIS)
The COVID-19 virus can induce premature cellular senescence in host cells by generating syncytial structures, multinucleated giant cells, resistant to apoptosis or efferocytosis as shown in Figure 3. For example, multinucleated large cancer cells, generated by ionizing radiation, were demonstrated to retain viability and resist elimination [67].
Figure 3. Exogenous viruses, including SARS-CoV-2, and endogenous viruses, such as HERVs, induce cell-cell fusion. Fusion starts with the formation of a fusion pore, followed by semi-fusion, cytoskeletal reorganization, and exchange of cytoplasm. TMEM16F is activated by the pore formation with calcium influx that flip phosphatidylserine (PS) from the inner to the outer leaflet of the cell membrane, a characteristic of damaged or senescent cells.
The SARS-CoV-2 virus can also activate HERVs that in return trigger fusion of host cells to each other, forming syncytia in which the pathogen can thrive undisturbed [68-70].
HERVs are viral fossils, comprising about 8% of the human genome, which have originated with ancient viral infections and were incorporated into the DNA. Some HERVs have been “domesticated” and have assumed physiological functions, one of which is placentation. For example, HERVs express several fusogens, including placental syncytins, which enable the formation of syncytiotrophoblast, and can be exploited by viruses to generate FIS [71]. Exogenous viruses, including SARS-CoV-2, have been demonstrated to activate HERVs, probably triggering FIS, a pathology documented in ME/CFS and long COVID [72,73].
The process of cell-cell fusion requires activation of molecular machinery that drives cytoskeletal remodeling to form giant cells. This molecular apparatus is comprised of TMEM16F, a calcium-dependent scramblase that flips PS from the inner leaflet of cell membrane to the cell surface where it signals readiness for apoptosis or fusion [74].
A Ca2+/Cal Modulin-dependent protein Kinase II (CaMKII) system was identified in the S protein of the SARS-CoV-2 virus, suggesting that TMEM16F may be activated by several mechanisms [75].
SARS-CoV-2 thrives in senescent cells
Due to dysfunctional NKCs, macrophage/microglia, virus-infected cells, may function as viral reservoirs, likely accounting for the protracted symptoms of long COVID [76-78].
This is substantiated by the following findings:
Multinucleated giant cells (syncytia) were found in SARS-CoV-2 infected individuals as well as after vaccination with messenger RNA (mRNA) therapeutics [79,80].
Viral RNA has been detected in postmortem monocytes and macrophages derived from COVID-19 patients, indicate that senescent, virus-infected cells, resist phagocytic elimination and can thrive for a long time [81].
The SARS-CoV-2 virus usurps the host NKCs via Non-structural Protein 1 (Nsp1), compromising efferocytosis further and contributing to the accumulation of senescent, virus-infected cells [82].
In contrast, SKF-38393, an allosteric modulator of Sigma-1 Receptors (Sig-1Rs) enhances macrophages/ microglia mediated clearance of damaged cells, while protecting the healthy ones, including the neurons [83,84]. Sig-1Rs also protect against SARS-CoV-2 infection, likely by augmenting efferocytosis and the elimination of virus-infected cells [85]. Furthermore candesartan, an inhibitor of intracellular Ca2+ influx, may block the activation of TMEM16F, decreasing cell-cell fusion and FIS.
Taken together, the SARS-CoV-2 virus induces premature cellular senescence and disrupts the elimination of senescent virus-infected cells. This triggers inflammation and disruption of the gut barrier and BBB, allowing the translocation of gut microbes into host tissues and organs. Conversely, SKF38393 enhances the efferocytosis of senescent cells, while candesartan inhibits cell-cell fusion, together eliminating viral reservoirs and the symptoms of long COVID.
Dysfunctional efferocytosis and long COVID
To maintain tissue homeostasis, millions of dead or dying cells need to be removed daily by professional and non-professional phagocytes, including macrophages and NKCs. Overproduction of senescent cells or defective efferocytosis promotes accumulation of virus- infected cells, generating latent inflammation and likely long COVID [86]. The binding of SARS-CoV-2 to its receptor, ACE-2, an ANG II degrading enzyme, contributes to ANG II accumulation. Upregulated ANG II promotes the release of cortisol and HMGB1, molecules associated with fatigue, IBD, muscle weakness, and malignant transformation [87-90]. In the gut, upregulation of cortisol and HMGB1 disrupts efferocytosis of IECs and ECs, contributing to microbial translocation. As gut microbes are not immunologically tolerated outside the GI tract, they trigger inflammatory responses associated with fatiguing illnesses [91]. Moreover, ANG II is toxic for mitochondria, organelles previously associated with fatiguing illnesses [92-94]. Furthermore, ANG II upregulates the Inter Cellular Adhesion Molecule 1 (ICAM-1), a transmembrane glycoprotein, overexpressed in many pathological states, including frailty, depression, and cognitive impairment [95,96]. ICAM-1 expression stimulates soluble ICAM-1 release in vitro and in vivo. AT1R blockade inhibits such endothelial effects of ANGII. Dysfunctional ICAM-1 has been shown to disrupt efferocytosis, further contributing to the accumulation of senescent cells [97]. Candesartan was demonstrated to inhibit the action of ANG II on ICAM-1, optimizing efferocytosis [98].
Taken together, excessive, unopposed ANG II disrupts mitochondria, and efferocytosis, leads to the accumulation of senescent cells and resultant inflammation that likely manifest as a fatiguing disorder.
Stress and biological barriers
Under physiological circumstances, intestinal barrier promotes absorption of nutrients, while preventing the migration of harmful substances, such as toxins or bacteria, into the systemic circulation. Psychological stress and pathogens were shown to disrupt the gut barrier, enabling translocation of microbes and/or their molecules into the body tissues and organs [99,100].
The Hypothalamic-Pituitary-Adrenal (HPA) axis can be activated by both psychological and biological stressors, including viral infections, highlighting the link between stress, cellular senescence, and bacteria [101,102].
Sterile inflammation refers to inflammasome activation by psychological and biological stressors, leading to numerous pathologies, including gut barrier dysfunction [103,104]. It is well established that chronic stress triggers cortisol release via HPA, contributing to various diseases. Although cortisol exhibits robust anti- inflammatory properties, novel studies have reported that chronic cortisol release can activate Nod-Like Receptor Protein 3 (NLRP3) inflammasome, inducing sterile inflammation that in turn disrupts the intestinal barrier [105,106]. For example, sterile inflammation in the GI tract has been associated with Major Depressive Disorder (MDD) and Parkinson’s disease, linking stress to NLRP3 activation documented in neuropsychiatric pathology [107-110].
HMGB1, upregulated by both biological and psychological stress, disrupts the gut barrier via a particular leitmotif, a Damage-Associated Molecular Patterns (DAMPs), highlighting a new target for microbial translocation disorders [111,112]. Like cortisol, HMGB1, was associated with premature cellular senescence, linking this molecule to sterile inflammation [113]. This may be significant as D1R partial agonists were shown to inhibit NLRP3 inflammasome directly, lowering all inflammatory responses, including the sterile inflammation [114]. In addition, chronic psychological stress has been shown to lower brain dopamine levels, suggesting that D1R partial agonists, including SKF38393, may reverse the detrimental effect of chronic stress. In this regard, cortisol-lowering properties of D1R partial agonists has been utilized in the treatment of Cushing syndrome, indicating that it can reverse both cortisol and HMGB1-mediated sterile inflammation [115]. Moreover, candesartan was demonstrated to lower HMGB1, indicating that along with SKF38393, it could decrease the effects of biological or psychological stress on the intestinal barrier [116,117]. These findings, likely significant for PTSD, may lead to the development of new strategies for this disorder that currently lacks adequate treatment.
Conclusion
Long COVID is marked by premature cellular senescence and clinical symptoms reminiscent of other fatiguing illnesses, including ME/CFS, CFS, and GWS. The SARS-CoV-2 virus is known for exploiting the human senescence program for ensuring undisturbed proliferation. However, senescence affecting the cells of biological barriers, including IECs, ECs, and lymphocytes, disrupt the tight junctions, enabling microbial translocation outside the GI tract. In addition, senescent cells upregulate plasma membrane ACE- 2 receptors that serve as SARS-CoV-2 entry portals, probably explaining why older individuals are at higher risk of COVID-19 critical illness. Together this data suggests that the virus may utilize senescent cells as reservoirs, accounting for viral signature in the monocytes and macrophages derived from deceased COVID-19 patients. Like HIV, latent SARS-CoV-2 in microglia, may explain the neuropsychiatric sequelae of long COVID. D1R partial agonist, SKF38393, and BBBcrossing ARB, candesartan, exert anti-inflammatory, pro-cognitive, and senolytic properties, suggesting efficacy in clearing viral reservoirs by decreasing the level of senescent cells.
References
- Krouwer VJ, Hekking LH, Langelaar-Makkinje M, Regan-Klapisz E, Post JA. Endothelial cell senescence is associated with disrupted cell-cell junctions and increased monolayer permeability. Vasc Cell 2012;4(1):12.
- Padia SH, Kemp BA, Howell NL, Keller SR, Gildea JJ, Carey RM. Mechanisms of dopamine D1 and angiotensin type 2 receptor interaction in natriuresis. Hypertension 2012;59(2):437-445.
- Jones-Tabah J, Mohammad H, Paulus EG, Clarke PB, Hébert TE. The signaling and pharmacology of the dopamine D1 receptor. Front Cell Neurosci 2022;15:806618.
- Nakagawa S, Sugiura M, Akitsuki Y, Hosseini SH, Kotozaki Y, Miyauchi CM, et al. Compensatory effort parallels midbrain deactivation during mental fatigue: An fMRI study. PLoS One 2013;8(2):e56606.
- Pall ML, Satterlee JD. Elevated nitric oxide/peroxynitrite mechanism for the common etiology of multiple chemical sensitivity, chronic fatigue syndrome, and posttraumatic stress disorder. Ann N Y Acad Sci 2001;933:323-329.
- Wilson T, Holt T, Greenhalgh T. Complexity and clinical care. BMJ 2001;323(7314):685-688.
- Nasi G, Ahmed T, Rasini E, Fenoglio D, Marino F, Filaci G, et al. Dopamine inhibits human CD8+ Treg function through D1-like dopaminergic receptors. J Neuroimmunol 2019;332:233-341.
- Larrayoz IM, Pang T, Benicky J, Pavel J, Sanchez-Lemus E, Saavedra JM. Candesartan reduces the innate immune response to lipopolysaccharide in human monocytes. J Hypertens 2009;27(12):2365-2376.
- Pedersen R, Johansson J, Salami A. Dopamine D1-signaling modulates maintenance of functional network segregation in aging. Aging Brain 2023;3:100079.
- Jiang Y, Gaur U, Cao Z, Hou ST, Zheng W. Dopamine D1-and D2-like receptors oppositely regulate lifespan via a dietary restriction mechanism in Caenorhabditis elegans. BMC Biol 2022;20(1):71.
- Huang W, Hickson LJ, Eirin A, Kirkland JL, Lerman LO. Cellular senescence: The good, the bad and the unknown. Nat Rev Nephrol 2022;18(10):611-627.
- Osorio C, Sfera A, Anton JJ, Thomas KG, Andronescu CV, Li E, et al. Virus-induced membrane fusion in neurodegenerative disorders. Front Cell Infect Microbiol 2022;12:845580.
- Gilbert DL, Dubow JS, Cunniff TM, Wanaski SP, Atkinson SD, Mahableshwarkar AR. Ecopipam for Tourette syndrome: A randomized trial. Pediatrics 2023;151(2):e2022059574.
- Kocienski P. Synthesis of ecopipam. Synfacts 2012;8(7):0704.
- Bezard E, Gray D, Kozak R, Leoni M, Combs C, Duvvuri S. Rationale and development of tavapadon, a D1/D5-selective partial dopamine agonist for the treatment of Parkinson’s disease. CNS Neurol Disord Drug Targets 2024;23(4):476-487.
- Isaacson SH, Hauser RA, Pahwa R, Gray D, Duvvuri S. Dopamine agonists in Parkinson’s disease: Impact of D1-like or D2-like dopamine receptor subtype selectivity and avenues for future treatment. Clin Park Relat Disord 2023;9:100212.
- Riesenberg R, Werth J, Zhang Y, Duvvuri S, Gray D. PF-06649751 efficacy and safety in early Parkinson’s disease: A randomized, placebo-controlled trial. Ther Adv Neurol Disord 2020;13:1756286420911296.
- Teng X, Chen S, Nie Y, Xiao P, Yu X, Shao Z, et al. Ligand recognition and biased agonism of the D1 dopamine receptor. Nat Commun 2022;13(1):3186.
- Glodzik L, Santisteban MM. Blood-brain barrier crossing renin-angiotensin system drugs: Considerations for dementia and cognitive decline. Hypertension 2021;78(3):644-646.
- Hu L, Li H, Zi M, Li W, Liu J, Yang Y, et al. Why senescent cells are resistant to apoptosis: An insight for senolytic development. Front Cell Dev Biol 2022;10:822816.
- Lynch SM, Guo G, Gibson DS, Bjourson AJ, Rai TS. Role of senescence and aging in SARS-CoV-2 infection and COVID-19 disease. Cells 2021;10(12):3367.
- Suzuki K, Susaki EA, Nagaoka I. Lipopolysaccharides and cellular senescence: Involvement in atherosclerosis. Int J Mol Sci 2022;23(19):11148.
- Lee S, Yu Y, Trimpert J, Benthani F, Mairhofer M, Richter-Pechanska P, et al. Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature 2021;599(7884):283-289.
- Seoane R, Vidal S, Bouzaher YH, El Motiam A, Rivas C. The interaction of viruses with the cellular senescence response. Biology (Basel) 2020;9(12):455.
- Schmitt CA, Tchkonia T, Niedernhofer LJ, Robbins PD, Kirkland JL, Lee S. COVID-19 and cellular senescence. Nat Rev Immunol 2023;23(4):251-263.
- Gal H, Krizhanovsky V. Cell fusion induced senescence. Aging (Albany NY) 2014;6(5):353-354.
- Lee JD, Menasche BL, Mavrikaki M, Uyemura MM, Hong SM, Kozlova N, et al. Differences in syncytia formation by SARS-CoV-2 variants modify host chromatin accessibility and cellular senescence via TP53. Cell Rep 2023;42(12):113478.
- Stallmach A, Quickert S, Puta C, Reuken PA. The gastrointestinal microbiota in the development of ME/CFS: A critical view and potential perspectives. Front Immunol 2024;15:1352744.
- Chuprin A, Gal H, Biron-Shental T, Biran A, Amiel A, Rozenblatt S, et al. Cell fusion induced by ERVWE1 or measles virus causes cellular senescence. Genes Dev 2013;27(21):2356-2366.
- Savina S, Zaydiner B. Cancer-related fatigue: Some clinical aspects. Asia Pac J Oncol Nurs 2019;6(1):7-9.
- Thong MS, Van Noorden CJ, Steindorf K, Arndt V. Cancer-related fatigue: Causes and current treatment options. Curr Treat Options Oncol. 2020;21(2):17.
- Servaes P, Gielissen MF, Verhagen S, Bleijenberg G. The course of severe fatigue in disease‐free breast cancer patients: A longitudinal study. Psychooncology. 2007;16(9):787-795.
- Arjmand MH. Elucidating the association between the upregulation of angiotensin type 1-receptors and the development of gastrointestinal malignancies. J Gastrointest Cancer 2021;52(2):399-406.
- Fan F, Tian C, Tao L, Wu H, Liu Z, Shen C, et al. Candesartan attenuates angiogenesis in hepatocellular carcinoma via downregulating AT1R/VEGF pathway. Biomed Pharmacother 2016;83:704-711.
- Abraham HM, White CM, White WB. The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf 2015;38(1):33-54.
- Johnson DE, Ochieng J, Evans SL. The growth inhibitory properties of a dopamine agonist (SKF 38393) on MCF-7 cells. Anticancer Drugs 1995;6(3):471-474.
- Sobczuk P, Lomiak M, Cudnoch-Jędrzejewska A. Dopamine D1 receptor in cancer. Cancers (Basel) 2020;12(11):3232.
- Dobryakova E, Genova HM, DeLuca J, Wylie GR. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front Neurol 2015;6:52.
- McCaffrey D, Lawther AJ, Weickert CS, Walker AK. Cancer activates microglia to the same extent as chronic stress throughout stress neurocircuitry in a mouse model of breast cancer. Psychoneuroendocrinology 2022;146:105938.
- Kavanagh E. Long Covid brain fog: A neuroinflammation phenomenon?. Oxf Open Immunol 2022;3(1):iqac007.
- Mavoungou E, Bouyou-Akotet MK, Kremsner PG. Effects of prolactin and cortisol on natural killer (NK) cell surface expression and function of human natural cytotoxicity receptors (NKp46, NKp44 and NKp30). Clin Exp Immunol 2005;139(2):287-296.
- Meeus M, Mistiaen W, Lambrecht L, Nijs J. Immunological similarities between cancer and chronic fatigue syndrome: The common link to fatigue?. Anticancer Res 2009;29(11):4717-4726.
- Wu Y, Luo X, Zhou Q, Gong H, Gao H, Liu T, et al. The disbalance of LRP1 and SIRPα by psychological stress dampens the clearance of tumor cells by macrophages. Acta Pharm Sin B 2022;12(1):197-209.
- Wang Y, Zhang W, Xu Y, Wu D, Gao Z, Zhou J, et al. Extracellular HMGB1 impairs macrophage-mediated efferocytosis by suppressing the Rab43-controlled cell surface transport of CD91. Front Immunol 2022;13:767630.
- Martin-Rodriguez O, Gauthier T, Bonnefoy F, Couturier M, Daoui A, Chagué C, et al. Pro-resolving factors released by macrophages after efferocytosis promote mucosal wound healing in inflammatory bowel disease. Front Immunol 2021;12:754475.
- Yamazaki Y, Baker DJ, Tachibana M, Liu CC, Van Deursen JM, Brott TG, et al. Vascular cell senescence contributes to blood–brain barrier breakdown. Stroke 2016;47(4):1068-1077.
- Jurewicz M, McDermott DH, Sechler JM, Tinckam K, Takakura A, Carpenter CB, et al. Human T and natural killer cells possess a functional renin-angiotensin system: Further mechanisms of angiotensin II–induced inflammation. J Am Soc Nephrol 2007;18(4):1093-1102.
- Zhao W, Huang Y, Liu Z, Cao BB, Peng YP, Qiu YH. Dopamine receptors modulate cytotoxicity of natural killer cells via cAMP-PKA-CREB signaling pathway. PLoS One 2013;8(6):e65860.
- Qie S, Ran Y, Lu X, Su W, Li W, Xi J, et al. Candesartan modulates microglia activation and polarization via NF-κB signaling pathway. Int J Immunopathol Pharmacol 2020;34:2058738420974900.
- Wallet C, De Rovere M, Van Assche J, Daouad F, De Wit S, Gautier V, et al. Microglial cells: The main HIV-1 reservoir in the brain. Front Cell Infect Microbiol 2019;9:362.
- Tayyar R, Ho D. Herpes simplex virus and varicella zoster virus infections in cancer patients. Viruses 2023;15(2):439.
- Masaldan S, Clatworthy SA, Gamell C, Meggyesy PM, Rigopoulos AT, Haupt S, et al. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol 2018;14:100-115.
- Samudyata N, Oliveira AO, Malwade S, Rufino de Sousa N, Goparaju SK, Gracias J, et al. SARS-CoV-2 promotes microglial synapse elimination in human brain organoids. Mol Psychiatry 2022;27(10):3939-3950.
- Sato T, Shapiro JS, Chang HC, Miller RA, Ardehali H. Aging is associated with increased brain iron through cortex-derived hepcidin expression. Elife 2022;11:e73456.
- Di Micco R, Krizhanovsky V, Baker D, d Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol 2021;22(2):75-95.
- Martin N, Zhu K, Czarnecka-Herok J, Vernier M, Bernard D. Regulation and role of calcium in cellular senescence. Cell Calcium 2023;110:102701.
- Proal AD, VanElzakker MB, Aleman S, Bach K, Boribong BP, Buggert M, et al. SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). Nat Immunol 2023;24(10):1616-1627.
- Victorelli S, Passos JF. Telomeres and cell senescence-size matters not. EBioMedicine 2017;21:14-20.
- Gioia U, Tavella S, Martínez-Orellana P, Cicio G, Colliva A, Ceccon M, et al. SARS-CoV-2 infection induces DNA damage, through CHK1 degradation and impaired 53BP1 recruitment, and cellular senescence. Nat Cell Biol 2023;25(4):550-564.
- Sepe S, Rossiello F, Cancila V, Iannelli F, Matti V, Cicio G, et al. DNA damage response at telomeres boosts the transcription of SARS‐CoV‐2 receptor ACE2 during aging. EMBO Rep 2022;23(2):e53658.
- Aguado J, Amarilla AA, Taherian Fard A, Albornoz EA, Tyshkovskiy A, Schwabenland M, et al. Senolytic therapy alleviates physiological human brain aging and COVID-19 neuropathology. Nat Aging 2023;3(12):1561-1575.
- Zhang X, Suda M, Zhu Y. Senolytics combat COVID-19 in aging. Nat Aging 2023;3(7):762-763.
- Zhao Y, Li Y, Li H, Shi S. Dopamine D1 receptor activation ameliorates ox-LDL-induced endothelial cell senescence via CREB/Nrf2 pathway. Exp Cell Res 2023;425(2):113542.
- Mirzayans R, Andrais B, Scott A, Wang YW, Kumar P, Murray D. Multinucleated giant cancer cells produced in response to ionizing radiation retain viability and replicate their genome. Int J Mol Sci. 2017;18(2):360.
- Blaise S, de Parseval N, Bénit L, Heidmann T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc Natl Acad Sci USA 2003;100(22):13013-13018.
- Dittmar T, Weiler J, Luo T, Hass R. Cell-cell fusion mediated by viruses and HERV-derived fusogens in cancer initiation and progression. Cancers 2021;13(21):5363.
- Nelson PN, Carnegie PR, Martin J, Ejtehadi HD, Hooley P, Roden D, et al. Demystified. Human endogenous retroviruses. Mol Pathol 2003;56(1):11-18.
- Denner J. Expression and function of endogenous retroviruses in the placenta. APMIS 2016;124(1-2):31-43.
- Giménez-Orenga K, Pierquin J, Brunel J, Charvet B, Martín-Martínez E, Perron H, et al. HERV-W ENV antigenemia and correlation of increased anti-SARS-CoV-2 immunoglobulin levels with post-COVID-19 symptoms. Front Immunol 2022;13:1020064.
- Rodrigues LS, da Silva Nali LH, Leal CO, Sabino EC, Lacerda EM, Kingdon CC, et al. HERV-K and HERV-W transcriptional activity in myalgic encephalomyelitis/chronic fatigue syndrome. Auto Immun Highlights 2019;10(1)-12.
- Whitlock JM, Chernomordik LV. Flagging fusion: Phosphatidylserine signaling in cell–cell fusion. J Biol Chem 2021;296:100411.
- Wenzhong L, Hualan L. COVID-19: The CaMKII-like system of S protein drives membrane fusion and induces syncytial multinucleated giant cells. Immunol Res 2021;69(6):496-519.
- Miron RJ, Bosshardt DD. Multinucleated giant cells: Good guys or bad guys?. Tissue Eng Part B Rev 2018;24(1):53-65.
- Rong Z, Mai H, Kapoor S, Puelles VG, Czogalla J, Schädler J, et al. SARS-CoV-2 spike protein accumulation in the skull-meninges-brain axis: Potential implications for long-term neurological complications in post-COVID-19. BioRxiv 2023:2023-04.
- Stadlmann S, Hein-Kuhnt R, Singer G. Viropathic multinuclear syncytial giant cells in bronchial fluid from a patient with COVID-19. J Clin Pathol 2020;73(9):607-608.
- Sung K, McCain J, King KR, Hong K, Aisagbonhi O, Adler ED, et al. Biopsy-proven giant cell myocarditis following the COVID-19 vaccine. Circ Heart Fail 2022;15(4):e009321.
- Buchrieser J, Dufloo J, Hubert M, Monel B, Planas D, Rajah MM, et al. Syncytia formation by SARS‐CoV‐2‐infected cells. EMBO J. 2020;39(23):e106267.
- Jalloh S, Olejnik J, Berrigan J, Nisa A, Suder EL, Akiyama H, et al. CD169-mediated restrictive SARS-CoV-2 infection of macrophages induces pro-inflammatory responses. PLoS Pathog 2022;18(10):e1010479.
- Lee MJ, Leong MW, Rustagi A, Beck A, Zeng L, Holmes S, et al. SARS-CoV-2 escapes direct NK cell killing through Nsp1-mediated downregulation of ligands for NKG2D. Cell Rep 2022;41(13):111892.
- Guo L, Chen Y, Zhao R, Wang G, Friedman E, Zhang A, Zhen X. Allosteric modulation of sigma‐1 receptors elicits anti‐seizure activities. Br J Pharmacol 2015;172(16):4052-4065.
- Zhang G, Li Q, Tao W, Qin P, Chen J, Yang H, et al. Sigma-1 receptor-regulated efferocytosis by infiltrating circulating macrophages/microglial cells protects against neuronal impairments and promotes functional recovery in cerebral ischemic stroke. Theranostics 2023;13(2):543-559.
- Vela JM. Repurposing sigma-1 receptor ligands for COVID-19 therapy?. Front Pharmacol 2020;11:582310.
- Gerlach BD, Ampomah PB, Yurdagul A, Liu C, Lauring MC, Wang X, et al. Efferocytosis induces macrophage proliferation to help resolve tissue injury. Cell Metab 2021;33(12):2445-2463.
- Kawano M, Nagata S. Efferocytosis and autoimmune disease. Int Immunol 2018;30(12):551-558.
- Kvivik I, Grimstad T, Jonsson G, Kvaløy JT, Omdal R. Anti-HMGB1 auto-Abs influence fatigue in patients with Crohn’s disease. Innate Immun 2021;27(4):286-293.
- Ho TL, Tang CH, Chang SL, Tsai CH, Chen HT, Su CM. HMGB1 promotes in vitro and in vivo skeletal muscle atrophy through an IL-18-dependent mechanism. Cells 2022;11(23):3936.
- Chen R, Zou J, Kang R, Tang D. The redox protein high-mobility group box 1 in cell death and cancer. Antioxid Redox Signal 2023;39(7-9):569-590.
- Komaroff AL. Inflammation correlates with symptoms in chronic fatigue syndrome. Proc Natl Acad Sci USA 2017;114(34):8914-8916.
- Morris G, Maes M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis 2014;29(1):19-36.
- Silva KA, Ghiarone T, Schreiber K, Grant D, White T, Frisard MI, et al. Angiotensin II suppresses autophagy and disrupts ultrastructural morphology and function of mitochondria in mouse skeletal muscle. J Appl Physiol 2019;126(6):1550-1562.
- Refolo G, Vescovo T, Piacentini M, Fimia GM, Ciccosanti F. Mitochondrial interactome: A focus on antiviral signaling pathways. Front Cell Dev Biol 2020;8:8.
- Baghai TC, Varallo-Bedarida G, Born C, Häfner S, Schüle C, Eser D, et al. Classical risk factors and inflammatory biomarkers: One of the missing biological links between cardiovascular disease and major depressive disorder. Int J Mol Sci 2018;19(6):1740.
- Nielsen HM, Londos E, Minthon L, Janciauskiene SM. Soluble adhesion molecules and angiotensin-converting enzyme in dementia. Neurobiol Dis 2007;26(1):27-35.
- Lee WJ, Chen LK, Liang CK, Peng LN, Chiou ST, et al. Soluble ICAM-1, independent of IL-6, is associated with prevalent frailty in community-dwelling elderly Taiwanese people. PLoS One 2016;11(6):e0157877.
- Wiesolek HL, Bui TM, Lee JJ, Dalal P, Finkielsztein A, Batra A, et al. Intercellular adhesion molecule 1 functions as an efferocytosis receptor in inflammatory macrophages. Am J Pathol 2020;190(4):874-885.
- Pastore L, Tessitore A, Martinotti S, Toniato E, Alesse E, Bravi MC, et al. Angiotensin II stimulates intercellular adhesion molecule-1 (ICAM-1) expression by human vascular endothelial cells and increases soluble ICAM-1 release in vivo. Circulation 1999;100(15):1646-1652.
- Varanoske AN, McClung HL, Sepowitz JJ, Halagarda CJ, Farina EK, Berryman CE, et al. Stress and the gut-brain axis: Cognitive performance, mood state, and biomarkers of blood-brain barrier and intestinal permeability following severe physical and psychological stress. Brain Behav Immun 2022;101:383-393.
- Mawdsley JE, Rampton DS. Psychological stress in IBD: New insights into pathogenic and therapeutic implications. Gut 2005;54(10):1481-1491.
- Silverman MN, Pearce BD, Biron CA, Miller AH. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol 2005;18(1):41-78.
- Chrousos GP, Zapanti ED. Hypothalamic-pituitary-adrenal axis in HIV infection and disease. Endocrinol Metab Clin North Am 2014;43(3):791-806.
- Iwata M, Ota KT, Duman RS. The inflammasome: Pathways linking psychological stress, depression, and systemic illnesses. Brain, behavior, and immunity. 2013;31:105-114.
- Fleshner M, Frank M, Maier SF. Danger signals and inflammasomes: Stress-evoked sterile inflammation in mood disorders. Neuropsychopharmacology 2017;42(1):36-45.
- Feng X, Zhao Y, Yang T, Song M, Wang C, Yao Y, et al. Glucocorticoid-driven NLRP3 inflammasome activation in hippocampal microglia mediates chronic stress-induced depressive-like behaviors. Front Mol Neurosci 2019;12:210.
- Bharti V, Tan H, Zhou H, Wang JF. Txnip mediates glucocorticoid-activated NLRP3 inflammatory signaling in mouse microglia. Neurochem Int 2019;131:104564.
- Shannon KM. Gut-derived sterile inflammation and Parkinson's disease. Front Neurol 2022;13:831090.
- Franklin TC, Xu C, Duman RS. Depression and sterile inflammation: Essential role of danger associated molecular patterns. Brain Behav Immun 2018;72:2-13.
- Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF. Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: a priming stimulus of microglia and the NLRP3 inflammasome. J Neurosci 2015;35(1):316-324.
- Wu TY, Liu L, Zhang W, Zhang Y, Liu YZ, Shen XL, et al. High-mobility group box-1 was released actively and involved in LPS induced depressive-like behavior. J Psychiatr Res 2015;64:99-106.
- Chen X, Zhao HX, Bai C, Zhou XY. Blockade of high-mobility group box 1 attenuates intestinal mucosal barrier dysfunction in experimental acute pancreatitis. Sci Rep 2017 ;7(1):6799.
- Gao X, Li F, Liu B, Wang Y, Wang Y, Zhou H. Cellular senescence in adrenocortical biology and its disorders. Cells 2021;10(12):3474.
- Lee JJ, Park IH, Kwak MS, Rhee WJ, Kim SH, Shin JS. HMGB1 orchestrates STING-mediated senescence via TRIM30α modulation in cancer cells. Cell Death Discov 2021;7(1):28.
- Jiang W, Li M, He F, Bian Z, Liu J, He Q, et al. Dopamine D1 receptor agonist A-68930 inhibits NLRP3 inflammasome activation and protects rats from spinal cord injury-induced acute lung injury. Spinal Cord 2016;54(11):951-956.
- Petrossians P, Thonnard AS, Beckers A. Medical treatment in Cushing’s syndrome: dopamine agonists and cabergoline. Neuroendocrinology 2010;92(Suppl. 1):116-119.
- Bloomfield MA, McCutcheon RA, Kempton M, Freeman TP, Howes O. The effects of psychosocial stress on dopaminergic function and the acute stress response. Elife 2019;8:e46797.
- Kikuchi K, Tancharoen S, Ito T, Morimoto-Yamashita Y, Miura N, Kawahara KI, et al. Potential of the angiotensin receptor blockers (ARBs) telmisartan, irbesartan, and candesartan for inhibiting the HMGB1/RAGE axis in prevention and acute treatment of stroke. Int J Mol Sci 2013;14(9):18899-18924.